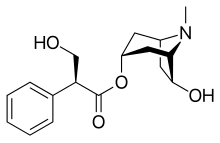
Anisodamine
 | |
| Clinical data | |
|---|---|
| Other names | 7β-hydroxyhyoscyamine |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.164.962 |
| Chemical and physical data | |
| Formula | C17H23NO4 |
| Molar mass | 305.374 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Anisodamine, also known as 7β-hydroxyhyoscyamine, is a mAChR anticholinergic and α1 adrenergic receptor antagonist used in the treatment of acute circulatory shock in China.[1] It is given orally or by injection, as a racemic mixture (racanisodamine) or as a hydrobromide salt of the natural enantiometer.[2] Eye drops at 0.5% concentration for slowing the progression of myopia is also available in China.[3]
Anisodamine is a naturally occurring tropane alkaloid found in some plants of the family Solanaceae including Datura.[4] Its Mandarin Chinese name 山莨菪碱 is given after Anisodus tanguticus (Chinese: 山莨菪; pinyin: shān làng dàng).[5]
In rodents, anisodamine is more "selective" in its action compared to atropine. It poorly passes the blood-brain barrier and binds brain mAChR less tightly. In rodents, it exhibits weaker CNS effects,[6] causes less mydriasis, but has approximately equal or slightly lower potency in blocking spasms and in reducing GI motility.[7] Chinese textbooks consider it to have a similar spectrum of effects on humans.[8] As a result, it (or rather, its synthetic racemic version) is widely used in China. It was added to China's national Essential Medicine List in 2012.[9]
See also
- Anisodine
- Atropine, used for similar cardiac and optamological purposes elsewhere
- Hyoscyamine
References
- ^ Varma DR, Yue TL (March 1986). "Adrenoceptor blocking properties of atropine-like agents anisodamine and anisodine on brain and cardiovascular tissues of rats". British Journal of Pharmacology. 87 (3): 587–594. doi:10.1111/j.1476-5381.1986.tb10201.x. PMC 1916562. PMID 2879586.
- ^ "Pharmacopoeia Search: "山莨菪碱"". 中国药典. Archived from the original on 2017-12-03.
- ^ "消旋山莨菪碱滴眼液防治少年儿童假性近视的疗效分析". 国际医药卫生导报 (in Chinese (China)). 14 (15): 67–68. 2008. doi:10.3760/cma.j.issn.1007-1245.2008.15.027. Archived from the original on 2023-03-27. Retrieved 2017-12-03.
- ^ Ye N, Li J, Gao C, Xie Y (August 2013). "Simultaneous determination of atropine, scopolamine, and anisodamine in Flos daturae by capillary electrophoresis using a capillary coated by graphene oxide". Journal of Separation Science. 36 (16): 2698–2702. doi:10.1002/jssc.201300304. PMID 23868645.
- ^ "消旋山莨菪碱" (in Chinese (China)). 中国药典. Archived from the original on 2017-12-03. Retrieved 3 December 2017.
- ^ Zhang WW, Song MK, Cui YY, Wang H, Zhu L, Niu YY, et al. (October 2008). "Differential neuropsychopharmacological influences of naturally occurring tropane alkaloids anisodamine versus scopolamine". Neuroscience Letters. 443 (3): 241–245. doi:10.1016/j.neulet.2008.07.048. PMID 18672024. S2CID 2730169.
- ^ Poupko JM, Baskin SI, Moore E (March 2007). "The pharmacological properties of anisodamine". Journal of Applied Toxicology. 27 (2): 116–121. doi:10.1002/jat.1154. PMID 17186568.
- ^ DAI TJ, YU T, eds. (2016). "第八章 作用于胆碱受体药物 [8 Agents acting on acetylcholine receptors]". 麻醉药理学 [Anesthesia Pharmacology] (in Chinese) (4 ed.). Beijing: 人民卫生出版社. p. 133. ISBN 978-7-117-22701-8.
- ^ Zhang Y, Zou J, Wan F, Peng F, Peng C (May 2023). "Update on the sources, pharmacokinetics, pharmacological action, and clinical application of anisodamine". Biomedicine & Pharmacotherapy. 161: 114522. doi:10.1016/j.biopha.2023.114522. PMID 37002581.
