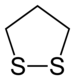
1,2-Dithiolane
| |||
| Names | |||
|---|---|---|---|
| IUPAC name 1,2-Dithiolane | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 102454 | |||
| ChEBI | |||
| ChemSpider | |||
| 1029938 | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C3H6S2 | |||
| Molar mass | 106.20 g·mol−1 | ||
| Related compounds | |||
Related compounds |
Ethane-1,2-dithiol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
1,2-Dithiolane is an organosulfur compound with the formula S2(CH2)3. It is also classified as a heterocycle derived from cyclopentane by replacing two methylene bridges (-CH
2- units) with a disulfide group. 1,3-Dithiolane is an isomer. The parent molecule is unimportant but substituted derivatives, especially lipoic acid and its derivatives, are often essential for life. Several occur naturally.[1]
The parent 1,2-dithiolane is the disulfide derived from 1,3-propanedithiol. It is however unstable with respect to oligomerization.[2] In general, 1,3-dithiols are superior reductants relative to monothiols.[3]
Natural occurrence
Many substituted 1,2-dithiolates are found in nature.[4] The most common is lipoic acid, a chiral dithiolane, which features a pentanoic acid substituent. It is essential for aerobic metabolism in mammals.
Some 1,2-dithiolane are found in some foods, such as asparagusic acid in asparagus.[5] The 4-dimethylamino derivative nereistoxin was the inspiration for insecticides that act by blocking the nicotinic acetylcholine receptor.[6]
Several alkyl-substituted 1,2-dithiolanes occur in the scent glands of skunks and related mammals. These include 3,3-dimethyl-, 3-propyl-, 3-ethyl-1,2-dithiolane, and others.[4]
- asparagusic acid
- nereistoxin, from which insecticides including cartap and bensultap were derived
- lipoic acid
- gerrardine, found in Cassipourea guianensis[4]
- charatoxin, found in chara globuluris[4]
Dithiolane-S-oxides

Many 1,2-dithiolanes can be oxidized to their S-oxides, which are chiral.[4]
References
- ^ Teuber, Lene (1990). "Naturally Occurring 1,2-Dithiolanes and 1,2,3-Trithianes. Chemical and Biological Properties". Sulfur Reports. 9 (4): 257–333. doi:10.1080/01961779008048732.
- ^ Goodrow, Marvin H.; Olmstead, Marilyn M.; Musker, W. Kenneth (1983). "Preparation and X-Ray Crystal Structure of the Cyclic Dimer of 1,2-Dithiolane: 1,2,6,7-Tetrathiacyclodecane". Phosphorus and Sulfur and the Related Elements. 16 (3): 299–302. doi:10.1080/03086648308080483.
- ^ Burns, John A.; Whitesides, George M. (1990). "Predicting the stability of cyclic disulfides by molecular modeling: Effective concentrations in thiol-disulfide interchange and the design of strongly reducing dithiols". Journal of the American Chemical Society. 112 (17): 6296–6303. doi:10.1021/ja00173a017.
- ^ a b c d e Teuber, Lene (1990). "Naturally Occurring 1,2-Dithiolanes and 1,2,3-Trithianes. Chemical and Biological Properties". Sulfur Reports. 9 (4): 257–333. doi:10.1080/01961779008048732.
- ^ Pelchat, M. L.; Bykowski, C.; Duke, F. F.; Reed, D. R. (2011). "Excretion and perception of a characteristic odor in urine after asparagus ingestion: A psychophysical and genetic study". Chemical Senses. 36 (1): 9–17. doi:10.1093/chemse/bjq081. PMC 3002398. PMID 20876394.
- ^ Casida, John E.; Durkin, Kathleen A. (2013). "Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects". Annual Review of Entomology. 58: 99–117. doi:10.1146/annurev-ento-120811-153645. PMID 23317040.




![gerrardine, found in Cassipourea guianensis[4]](https://upload.wikimedia.org/wikipedia/commons/thumb/b/b6/Gerrardine.svg/120px-Gerrardine.svg.png)
![charatoxin, found in chara globuluris[4]](https://upload.wikimedia.org/wikipedia/commons/thumb/b/b7/Charatoxin.svg/66px-Charatoxin.svg.png)