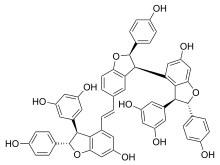
Vitisin C
Names
Preferred IUPAC name (22 S ,23 S ,32 R ,33 S ,4E ,62 S ,63 S )-22 ,32 ,62 -Tris(4-hydroxyphenyl)-22 ,23 ,32 ,33 ,62 ,63 -hexahydro-2(3,4),3(3,5),6(4,3)-tris([1]benzofurana)-1,7(1)-dibenzenaheptaphan-4-ene-13 ,15 ,26 ,66 ,73 ,75 -hexol
Identifiers
ChEMBL
ChemSpider
UNII
InChI=1S/C56H42O12/c57-35-10-4-29(5-11-35)54-50(33-19-38(60)23-39(61)20-33)49-32(18-42(64)26-47(49)67-54)3-1-28-2-16-46-44(17-28)52(56(66-46)31-8-14-37(59)15-9-31)45-25-43(65)27-48-53(45)51(34-21-40(62)24-41(63)22-34)55(68-48)30-6-12-36(58)13-7-30/h1-27,50-52,54-65H/b3-1+/t50-,51-,52+,54+,55+,56-/m0/s1
Y Key: WZKKRZSJTLGPHH-WHSOPTDBSA-N
Y InChI=1/C56H42O12/c57-35-10-4-29(5-11-35)54-50(33-19-38(60)23-39(61)20-33)49-32(18-42(64)26-47(49)67-54)3-1-28-2-16-46-44(17-28)52(56(66-46)31-8-14-37(59)15-9-31)45-25-43(65)27-48-53(45)51(34-21-40(62)24-41(63)22-34)55(68-48)30-6-12-36(58)13-7-30/h1-27,50-52,54-65H/b3-1+/t50-,51-,52+,54+,55+,56-/m0/s1
Key: WZKKRZSJTLGPHH-WHSOPTDBBK
OC(C=C1)=CC=C1[C@@H](O2)[C@@H](C3=CC(O)=CC(O)=C3)C4=C2C=C(O)C=C4/C=C/C5=CC([C@H](C6=C([C@H](C7=CC(O)=CC(O)=C7)[C@@H](C8=CC=C(O)C=C8)O9)C9=CC(O)=C6)[C@H](C%10=CC=C(O)C=C%10)O%11)=C%11C=C5
Oc1ccc(cc1)[C@H]4Oc2cc(O)cc(c2[C@@H]4c3cc(O)cc(O)c3)\C=C\c%10ccc%11O[C@@H](c5ccc(O)cc5)[C@@H](c7cc(O)cc8O[C@H](c6ccc(O)cc6)[C@H](c78)c9cc(O)cc(O)c9)c%11c%10
Properties
C 56 H 42 O 12
Molar mass
−1
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Vitisin C is a hydroxystilbenoid . It is a resveratrol tetramer found in plants of the genus Vitis [ 1] [ 2]
References
^ Ito, J (1996). "Absolute structures of new hydroxystilbenoids, vitisin C and viniferal, from Vitis vinifera 'Kyohou'Tetrahedron . 52 (30): 9991–9998. doi :10.1016/0040-4020(96)00543-1 . ^ Seya, K; Furukawa, K; Taniguchi, S; Kodzuka, G; Oshima, Y; Niwa, M; Motomura, S (2003). "Endothelium-dependent vasodilatory effect of vitisin C, a novel plant oligostilbene from Vitis plants (Vitaceae), in rabbit aorta". Clinical Science . 105 (1): 73–9. doi :10.1042/CS20020288 . PMID 12605596 . S2CID 41885739 .
External links
Dimers Trimers Tetramers: Higher polymers Oligomeric forms
Dimers Trimers Tetramers Pentamers Hexamers Higher polymers
Glycosides or conjugates