Suramin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Antrypol, 309 Fourneau, Bayer 205, others |
| AHFS/Drugs.com | Drugs.com archive |
| Routes of administration | by injection only |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.145 |
| Chemical and physical data | |
| Formula | C51H40N6O23S6 |
| Molar mass | 1297.26 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Suramin is a medication used to treat African sleeping sickness and river blindness.[1][2] It is the treatment of choice for sleeping sickness without central nervous system involvement.[3] It is given by injection into a vein.[4]
Suramin causes a fair number of side effects.[4] Common side effects include nausea, vomiting, diarrhea, headache, skin tingling, and weakness.[2] Sore palms of the hands and soles of the feet, trouble seeing, fever, and abdominal pain may also occur.[2] Severe side effects may include low blood pressure, decreased level of consciousness, kidney problems, and low blood cell levels.[4] It is unclear if it is safe when breastfeeding.[2]
Suramin was made at least as early as 1916.[5] It is on the World Health Organization's List of Essential Medicines.[6] In the United States it can be acquired from the Centers for Disease Control (CDC).[3] In regions of the world where the disease is common suramin is provided for free by the World Health Organization (WHO).[7]
Medical uses
Suramin is used for treatment of human sleeping sickness caused by trypanosomes.[1] Specifically, it is used for treatment of first-stage African trypanosomiasis caused by Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense without involvement of central nervous system.[8][9] It is considered first-line treatment for Trypanosoma brucei rhodesiense, and second-line treatment for early-stage Trypanosoma brucei gambiense, where pentamidine is recommended as first line.[9]
It has been used in the treatment of river blindness (onchocerciasis).[2]
Pregnancy and breastfeeding
It is unknown whether it is safe for the baby when a woman takes it while breastfeeding.[2]
Adverse reactions
The most frequent adverse reactions are nausea, vomiting, diarrhea, abdominal pain, and a feeling of general discomfort. It is also common to experience various sensations in the skin, from crawling or tingling sensations, tenderness of palms and the soles, and numbness of hands, arm, legs or feet.[10] Other skin reactions include skin rash, swelling and stinging sensation.[10] Suramin can also cause loss of appetite and irritability.[10] Suramin causes non-harmful changes in urine during use, specifically making the urine cloudy.[10] It may exacerbate kidney disease.[11]
Less common side effects include extreme fatigue, ulcers in the mouth, and painful tender glands in the neck, armpits and groin.[10] Suramin uncommonly affects the eyes causing watery eyes, swelling around the eyes, photophobia, and changes or loss of vision.[10]
Rare side effects include hypersensitivity reactions causing difficulty breathing. Other rare systemic effects include decreased blood pressure, fever, rapid heart rate, and convulsions.[10] Other rare side effects include symptoms of liver dysfunction such as tenderness in upper abdomen, jaundice in eyes and skin, unusual bleeding or bruising.[10]
Suramin has been applied clinically to HIV/AIDS patients resulting in a significant number of fatal occurrences and as a result the application of this molecule was abandoned for this condition.[12]
Pharmacology
Pharmacokinetics
Suramin is not orally bioavailable and must be given intravenously. Intramuscular and subcutaneous administration could result in local tissue inflammation or necrosis [citation needed]. Suramin is approximately 99-98% protein bound in the serum and has a half-life of 41–78 days average of 50 days; however, the pharmacokinetics of suramin can vary substantially between individual patients. Suramin does not distribute well into cerebral spinal fluid and its concentration in the tissues is equivalently lower than its concentration in the plasma. Suramin is not extensively metabolized and about 80% is eliminated via the kidneys.[11]
Mechanism of action
The mechanism of action for suramin is unclear, but it is thought that parasites are able to selectively uptake suramin via receptor-mediated endocytosis of drug that is bound to low-density lipoproteins and, to a lesser extent, other serum proteins.[11] Once inside parasites, suramin combines with proteins, especially trypanosomal glycolytic enzymes, to inhibit energy metabolism.[13]
Chemistry
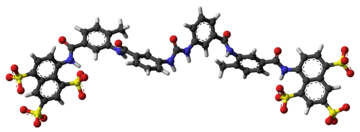
The molecular formula of suramin is C51H40N6O23S6. It is a symmetric molecule in the center of which lies a urea (NH–CO–NH) functional group. Suramin contains six aromatic systems – four benzene rings, sandwiched by a pair of naphthalene moieties – plus four amide functional groups (in addition to the urea) and six sulfonic acid groups. When given as a medication, it is usually delivered as the sodium sulfonate salt as this formulation is water-soluble, though it does deteriorate rapidly in air.[11]
The synthesis of suramin itself and structural analogs is by successive formation of the amide bonds from their corresponding amine (aniline) and carboxyl (as acyl chloride) components. Various routes to these compounds have been developed, including starting from separate naphthalene structures and building towards an eventual unification by formation of the urea[14][15] or starting with a urea and appending successive groups.[16]
History
Suramin was first made by the chemists Oskar Dressel, Richard Kothe and Bernhard Heymann at Bayer AG laboratories in Elberfeld, after research on a series of urea-like compounds. The drug is still sold by Bayer under the brand name Germanin. The chemical structure of suramin was kept secret by Bayer for commercial and strategic reasons, but it was elucidated and published in 1924 by Ernest Fourneau and his team at the Pasteur Institute.[17]: 378–379 [18]
Research
It is also used as a research reagent to inhibit the activation of heterotrimeric G proteins in a variety of GPCRs with varying potency. It prevents the association of heteromeric G proteins and therefore the receptors guanine exchange functionality (GEF). With this blockade the GDP will not release from the Gα subunit so it can not be replaced by a GTP and become activated. This has the effect of blocking downstream G protein mediated signaling of various GPCR proteins including rhodopsin, the A1 adenosine receptor, the D2 receptor,[19] the P2 receptor,[20][21] and ryanodine receptors.[22] Suramin is also an inhibitor of ABC-type[23] and P-type[24] ATPases, which acts competitively with ATP.
Suramin was studied as a possible treatment for prostate cancer in a clinical trial.[25]
Suramin has been studied in a mouse model of autism and in a small phase I/II human trial.[26][27][28][29] Results from a randomized clinical study found no statistically significant effects of suramin (in either 10mg or 20mg doses) versus placebo on boys with moderate to severe autism spectrum disorder.[30]
Suramin is a reversible and competitive protein tyrosine phosphatase (PTPases) inhibitor, also is the potent inhibitor of sirtuins, purified topoisomerase II and SARS-CoV-2 RNA-dependent RNA polymerase (RdRp).[31]
References
- ^ a b "Suramin Injection Advanced Patient Information". Drugs.com. 3 January 2020. Retrieved 11 January 2020.
- ^ a b c d e f "Micromedex Detailed Drug Information for the Consumer: Suramin (Injection route)". PubMed Health. 1 November 2016. Archived from the original on 8 September 2017.
- ^ a b "Our Formulary Infectious Diseases Laboratories CDC". www.cdc.gov. 22 September 2016. Archived from the original on 16 December 2016. Retrieved 30 November 2016.
- ^ a b c Zuckerman JN (2002). Principles and Practice of Travel Medicine. John Wiley & Sons. p. 113. ISBN 9780471490791. Archived from the original on 30 November 2016.
- ^ Mehlhorn H (2008). Encyclopedia of Parasitology: A-M. Springer Science & Business Media. p. 475. ISBN 9783540489948. Archived from the original on 30 November 2016.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "Trypanosomiasis, human African (sleeping sickness)". World Health Organization. February 2016. Archived from the original on 4 December 2016. Retrieved 7 December 2016.
- ^ "CDC Infectious Diseases Laboratory: Our Formulary". CDC. Archived from the original on 8 November 2016. Retrieved 8 November 2016.
- ^ a b Kappagoda S, Singh U, Blackburn BG (June 2011). "Antiparasitic therapy". Mayo Clinic Proceedings. 86 (6): 561–583. doi:10.4065/mcp.2011.0203. PMC 3104918. PMID 21628620.
- ^ a b c d e f g h "Suramin Injection Advanced Patient Information - Drugs.com". Archived from the original on 8 November 2016. Retrieved 8 November 2016.
- ^ a b c d Phillips MA, Stanley Jr SL (2011). "Chapter 50: Chemotherapy of Protozoal Infections: Amebiasis, Giardiasis, Trichomoniasis, Trypanosomiasis, Leishmaniasis, and Other Protozoal Infections". In Brunton LL, Chabner BA, Knollmann BC (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics (12th ed.). McGraw Hill. pp. 1437–1438. ISBN 9780071769396.
- ^ Kaplan LD, Wolfe PR, Volberding PA, Feorino P, Levy JA, Abrams DI, et al. (March 1987). "Lack of response to suramin in patients with AIDS and AIDS-related complex". The American Journal of Medicine. 82 (3 Spec No): 615–620. doi:10.1016/0002-9343(87)90108-2. PMID 3548350.
- ^ Moore TA (2015). "246e: Agents Used to Treat Parasitic Infections". In Kasper DL, et al. (eds.). Harrison's Principles of Internal Medicine (19th ed.). McGraw-Hil. ISBN 9780071802161.
- ^ Kassack MU, Braun K, Ganso M, Ullmann H, Nickel P, Böing B, et al. (April 2004). "Structure-activity relationships of analogues of NF449 confirm NF449 as the most potent and selective known P2X1 receptor antagonist". European Journal of Medicinal Chemistry. 39 (4): 345–357. doi:10.1016/j.ejmech.2004.01.007. PMID 15072843.
- ^ Ullmann H, Meis S, Hongwiset D, Marzian C, Wiese M, Nickel P, et al. (November 2005). "Synthesis and structure-activity relationships of suramin-derived P2Y11 receptor antagonists with nanomolar potency". Journal of Medicinal Chemistry. 48 (22): 7040–7048. doi:10.1021/jm050301p. PMID 16250663.
- ^ McGeary RP, Bennett AJ, Tran QB, Prins J, Ross BP (2009). "An 'inside-out' approach to suramin analogues". Tetrahedron. 65 (20): 3990–3997. doi:10.1016/j.tet.2009.03.033.
- ^ Sneader W (2005). Drug Discovery: A History. John Wiley & Sons. ISBN 9780471899792.
- ^ Fourneau E, Théfouël VJ, Vallée J (1924). "Sur une nouvelle série de médicaments trypanocides". Comptes Rendus des Séances de l'Académie des Sciences. 178: 675.
- ^ Beindl W, Mitterauer T, Hohenegger M, Ijzerman AP, Nanoff C, Freissmuth M (August 1996). "Inhibition of receptor/G protein coupling by suramin analogues". Molecular Pharmacology. 50 (2): 415–423. PMID 8700151. Archived from the original on 8 September 2017.
- ^ Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, et al. (September 2006). "International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy". Pharmacological Reviews. 58 (3): 281–341. doi:10.1124/pr.58.3.3. PMC 3471216. PMID 16968944.
- ^ Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Séguéla P, et al. (March 2001). "International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits". Pharmacological Reviews. 53 (1): 107–118. PMID 11171941. Archived from the original on 18 November 2016.
- ^ Wolner I, Kassack MU, Ullmann H, Karel A, Hohenegger M (October 2005). "Use-dependent inhibition of the skeletal muscle ryanodine receptor by the suramin analogue NF676". British Journal of Pharmacology. 146 (4): 525–533. doi:10.1038/sj.bjp.0706359. PMC 1751178. PMID 16056233.
- ^ Buxbaum E (October 1999). "Co-operative binding sites for transported substrates in the multiple drug resistance transporter Mdr1". European Journal of Biochemistry. 265 (1): 64–70. doi:10.1046/j.1432-1327.1999.00644.x. PMID 10491158.
- ^ Fortes PA, Ellory JC, Lew VL (August 1973). "Suramin: a potent ATPase inhibitor which acts on the inside surface of the sodium pump". Biochimica et Biophysica Acta (BBA) - Biomembranes. 318 (2): 262–72. doi:10.1016/0005-2736(73)90119-3. PMID 4355468.
- ^ Ahles TA, Herndon JE, Small EJ, Vogelzang NJ, Kornblith AB, Ratain MJ, et al. (November 2004). "Quality of life impact of three different doses of suramin in patients with metastatic hormone-refractory prostate carcinoma: results of Intergroup O159/Cancer and Leukemia Group B 9480". Cancer. 101 (10): 2202–2208. doi:10.1002/cncr.20655. PMID 15484217. S2CID 29107328.
- ^ LaFee S, Buschman H (26 May 2017). "Researchers Studying Century-Old Drug in Potential New Approach to Autism". UC San Diego Health. Archived from the original on 1 June 2017.
- ^ Naviaux JC, Schuchbauer MA, Li K, Wang L, Risbrough VB, Powell SB, et al. (June 2014). "Reversal of autism-like behaviors and metabolism in adult mice with single-dose antipurinergic therapy". Translational Psychiatry. 4 (6): e400. doi:10.1038/tp.2014.33. PMC 4080315. PMID 24937094.
- ^ Naviaux RK, Curtis B, Li K, Naviaux JC, Bright AT, Reiner GE, et al. (July 2017). "Low-dose suramin in autism spectrum disorder: a small, phase I/II, randomized clinical trial". Annals of Clinical and Translational Neurology. 4 (7): 491–505. doi:10.1002/acn3.424. PMC 5497533. PMID 28695149.
- ^ "Q and A - Suramin and Autism". UC Health - UC San Diego. Retrieved 27 July 2021.
- ^ Hough D, Mao AR, Aman M, Lozano R, Smith-Hicks C, Martinez-Cerdeno V, et al. (November 2023). "Randomized clinical trial of low dose suramin intravenous infusions for treatment of autism spectrum disorder". Annals of General Psychiatry. 22 (1): 45. doi:10.1186/s12991-023-00477-8. PMC 10626700. PMID 37932739.
- ^ "Suramin sodium salt". Selleck Chemicals.
Further reading
- Zhang YL, Keng YF, Zhao Y, Wu L, Zhang ZY (May 1998). "Suramin is an active site-directed, reversible, and tight-binding inhibitor of protein-tyrosine phosphatases". The Journal of Biological Chemistry. 273 (20): 12281–12287. doi:10.1074/jbc.273.20.12281. PMID 9575179.
External links
- Suramin sodium National Cancer Institute
