Titration: Difference between revisions
12.119.193.206 (talk) |
No edit summary |
||
| Line 7: | Line 7: | ||
Many methods can be used to indicate the endpoint of a reaction; titrations often use visual indicators (the reactant mixture changes colour). In simple [[acid-base titration]]s a [[pH indicator]] may be used, such as [[phenolphthalein]], which turns (and stays) pink when a certain pH is reached or exceeded. [[Methyl orange]] can also be used, which is red in acids and yellow in alkalis. |
Many methods can be used to indicate the endpoint of a reaction; titrations often use visual indicators (the reactant mixture changes colour). In simple [[acid-base titration]]s a [[pH indicator]] may be used, such as [[phenolphthalein]], which turns (and stays) pink when a certain pH is reached or exceeded. [[Methyl orange]] can also be used, which is red in acids and yellow in alkalis. |
||
However, please take note that not every titration requires an indicator. For example, an oxidisation-reduction titration involving Potassium Permanganate (Purple) does not require an indicator as, when Potassium Permanganate is oxidised, it turns colorless. |
|||
Due to the logarithmic nature of the pH curve, the transitions are generally extremely sharp, and thus a single drop of titrant just before the ''endpoint'' can change the pH by several points — leading to an immediate colour change in the [[indicator]]. That said, there is a slight difference between the change in indicator color and the actual equivalence point of the titration. This error is referred to as an indicator error, and it is indeterminate. |
Due to the logarithmic nature of the pH curve, the transitions are generally extremely sharp, and thus a single drop of titrant just before the ''endpoint'' can change the pH by several points — leading to an immediate colour change in the [[indicator]]. That said, there is a slight difference between the change in indicator color and the actual equivalence point of the titration. This error is referred to as an indicator error, and it is indeterminate. |
||
Revision as of 12:17, 5 September 2006
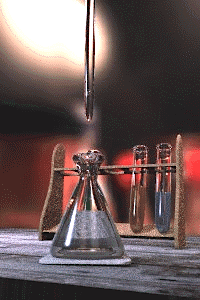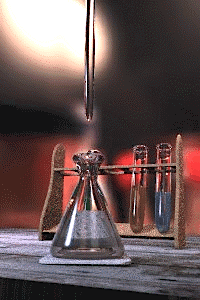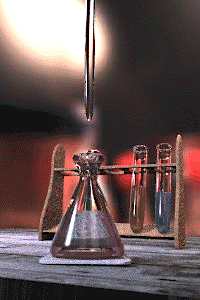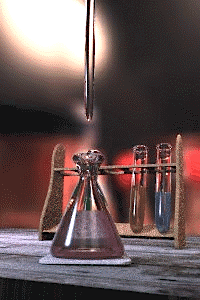
Titration is a standard laboratory method of quantitative/chemical analysis which can be used to determine the concentration of a known reactant. Because volume measurements play a key role in titration, it is also known as volumetric analysis. A reagent, called the titrant, of known concentration (a standard solution) and volume is used to react with a measured quantity of reactant (Analyte). Using a calibrated burette to add the titrant, it is possible to determine the exact amount that has been consumed when the endpoint is reached. The endpoint is the point at which the titration is stopped. This is classically a point at which the number of moles of titrant is equal to the number of moles of analyte, or some multiple thereof (as in di- or tri- protic acids). In the classic strong acid-strong base titration the endpoint of a titration is when the pH of the reactant is just about equal to 7, and often when the solution permanently changes color due to an indicator. There are however many different types of titrations (see below).
Many methods can be used to indicate the endpoint of a reaction; titrations often use visual indicators (the reactant mixture changes colour). In simple acid-base titrations a pH indicator may be used, such as phenolphthalein, which turns (and stays) pink when a certain pH is reached or exceeded. Methyl orange can also be used, which is red in acids and yellow in alkalis.
However, please take note that not every titration requires an indicator. For example, an oxidisation-reduction titration involving Potassium Permanganate (Purple) does not require an indicator as, when Potassium Permanganate is oxidised, it turns colorless.
Due to the logarithmic nature of the pH curve, the transitions are generally extremely sharp, and thus a single drop of titrant just before the endpoint can change the pH by several points — leading to an immediate colour change in the indicator. That said, there is a slight difference between the change in indicator color and the actual equivalence point of the titration. This error is referred to as an indicator error, and it is indeterminate.
Etymology
The word "titration" comes from the Latin "titalus", meaning inscription or title. The French word, titre, also from this origin, means rank. Titration is by definition the determination of rank or concentration of a solution, so it corresponds.
Preparing a sample for titration
Titrations are performed "in solution". If the sample isn't a liquid or solution, the samples must be dissolved. If the analyte is very concentrated in the sample, it might be useful to dilute the sample.
Although the vast majority of titrations are carried out in aqueous solution, other solvents such as glacial acetic acid or ethanol (in petrochemistry) are used for special purposes.
A measured amount of the sample can be given in the flask and then be dissolved or diluted. The mathematical result of the titration can be calculated directly with the measured amount. Sometimes the sample is dissolved or diluted beforehand and a measured amount of the solution is used for titration. In this case the dissolving or diluting must be done accurately with a known coefficient because the mathematical result of the titration must be multiplied with this factor.
A lot of titrations require buffering to maintain a certain pH for the reaction. Therefore buffer solutions are added to the reactant solution in the flask.
Some titrations require "masking" of a certain ion. This can be necessary when two reactants in the sample would react with the titrant and only one of them must be analysed, or when the reaction would be disturbed or inhibited by this ion. In this case another solution is added to the sample which "masks" the unwanted ion (for instance by a weak binding with it or even forming a solid insoluble substance with it).
Some redox reactions may require heating the solution with the sample and titration while the solution is still hot (to increase the reaction rate).
Procedure
N.B. Before starting, make sure that all of your glassware—especially the burette—is clean and dry. Alternatively, you can rinse out the glassware with the solution that is to be stored in the particular piece, as this will coat the glassware with the solution as to not affect the concentration of the solution.
- Accurately measure a volume of the reactant (this is usually done with a pipette) into a beaker or Erlenmeyer flask.
- Add a suitable indicator to the flask. As small an amount as possible is preferable; this will mitigate the effect of indicator on the pH.
- Flush through the burette first with tap water, then distilled water and finally the titrant.
- Pour the titrant into the burette, enlisting the help of a filter funnel, if necessary. Remove the filter funnel, if used. This is important as leftover solution from the funnel can continue flowing into the burette, affecting your reading.
- IMPORTANT: Ensure that there is no air-space at the tip of the burette. This can be seen as a bubble in between the tap and the the end tip of the burette. (To remedy this, turn the tap and allow a fast jet of solution to flow through, shaking the burette vigorously if necessary, collecting the titrant solution in a separate beaker as waste.)
- Read the start-point of the liquid on the burette.
- Turn the tap of the burette to allow the titrant to fall slowly into the reactant. For best results, ensure that the end tip of the burette is inside the flask or beaker (but not touching the solution!). Swirl the flask with the other hand or with a magnetic "flea".
- The indicator should change colour as the titrant is added, but then quickly return to its original colour.
- As the end-point is approached, the indicator takes longer to turn back to its starting colour. Add the titrant more slowly at this point (one drop at a time).
- When the indicator remains at its end colour, the reaction has reached the end point. Measure the amount of titrant liquid remaining, as shown on the scale of the burette. As is standard for measuring any liquids in the laboratory, measure from the bottom of the meniscus if it is concave, and from the top if it is convex. The volume used (endpoint volume) is the difference of the two readings (initial and final).
- Repeat, then average the volumes.
Once the number of moles of reactant that have been neutralised has been determined then it is easy to calculate the concentration in moles per litre.
in the above formula
C represents the concentration (Molarity)
N represents the number of moles (mol), and
V represents the volume of the solution (L).
Titration curves
Titrations are often recorded on titration curves, whose compositions are generally identical: the independent variable is the volume of the titrant, while the dependent variable is the pH of the solution (which changes depending on the composition of the two solutions). The equivalence point is a significant point on the graph (the point at which all of the starting solution, usually an acid, has been neutralized by the titrant, usually a base). It can be calculated precisely by finding the second derivative of the titration curve and computing the points of inflection (where the graph changes concavity); however, in most cases, simple visual inspection of the curve will suffice (in the curve given to the right, the only immediately visible equivalence point (actually the second equivalence point) occurs after roughly 5 mL of MMH has been titrated with the citric acid solution) Therefore, to calculate the other two Pka values, one must take the seen pKa value and divide this volume into the respective amounts to find the 2 other equivalence points, and therefore half-equivalence points.
In monoprotic acids, the point halfway between the beginning of the curve (before any titrant has been added) and the equivalence point is significant: at that point, the concentrations of the two solutions (the titrant and the original solution) are equal. Therefore, the Henderson-Hasselbalch equation can be solved in this manner:
Therefore, one can easily find the acid dissociation constant of the monoprotic acid by finding the pH of the point halfway between the beginning of the curve and the equivalence point, and solving the simplified equation. In the case of the sample curve, the Ka would be approximately from visual inspection (the actual Ka2 is ).
For polyprotic acids, calculating the acid dissociation constants is only marginally more difficult: the first acid dissociation constant can be calculated the same way as it would be calculated in a monoprotic acid. The second acid dissociation constant, however, is the point halfway between the first equivalence point and the second equivalence point (and so on for acids that release more than two protons, such as phosphoric acid).
Types
Titrations can be classified by the type of reaction, or by the method used to determine the endpoint. In many cases, several different methods could be used to detect the endpoint of a given type of reaction.
By Reaction
Different types of titration reaction include:
- Acid-base titration is based on the neutralization reaction between the analyte and an acidic or basic titrant. These most commonly use a pH indicator, a pH meter, or a conductance meter to determine the endpoint.
- A Redox titration is based on an oxidation-reduction reaction between the analyte and titrant. These most commonly use a potentiometer or a redox indicator to determine the endpoint. Frequently either the reactants or the titrant have a colour intense enough that an additional indicator is not needed.
- A Complexometric titration is based on the formation of a complex between the analyte and the titrant. The chelating agent EDTA is very commonly used to titrate metal ions in solution. These titrations generally require specialized indicators that form weaker complexes with the analyte. A common example is Eriochrome Black T for the titration of calcium and magnesium ions.
- A form of titration can also be used to determine the concentration of a virus or bacterium. The original sample is diluted (in some fixed ratio, such as 1:1, 1:2, 1:4, 1:8, etc.) until the last dilution does not give a positive test for the presence of the virus. This value, the titre, may be based on TCID50, EID50, ELD50, LD50 or pfu. This procedure is more commonly known as an assay.
By Detection
Different methods to determine the endpoint include:
- pH indicator: This is a substance that changes colour in response to a chemical change. An acid-base indicator (e.g., phenolphthalein) changes colour depending on the pH. Redox indicators are also frequently used. A drop of indicator solution is added to the titration at the start; when the colour changes the endpoint has been reached.
- A potentiometer can also be used. This is an instrument which measures the electrode potential of the solution. These are used for titrations based on a redox reaction; the potential of the working electrode will suddenly change as the endpoint is reached.
- pH meter: This is a potentiometer which uses an electrode whose potential depends on the amount of H+ ion present in the solution. (This is an example of an ion selective electrode. This allows the pH of the solution to be measured throughout the titration. At the end point there will be a sudden change in the measured pH. It can be more accurate than the indicator method, and is very easily automated.
- Conductance: The conductivity of a solution depends on the ions that are present in it. During many titrations, the conductivity changes significantly. (For instance, during an acid-base titration, the H+ and OH- ions react to form neutral H2O. This changes the conductivity of the solution.) The total conductance of the solution depends also on the other ions present in the solution (such as counter ions). Not all ions contribute equally to the conductivity; this also depends on the mobility of each ion and on the total concentration of ions (ionic strength). Thus, predicting the change in conductivity is harder than measuring it.
- Colour change: In some reactions, the solution changes colour without any added indicator. This is often seen in redox titrations, for instance, when the different oxidation states of the product and reactant produce different colours.
- Precipitation: If the reaction forms a solid, then a precipitate will form during the titration. A classic example is the reaction between Ag+ and Cl- to form the very insoluble salt AgCl. Surprisingly, this usually makes it difficult to determine the endpoint precisely. As a result, precipitation titrations often have to be done as "back" titrations (see below).
- An isothermal titration calorimeter uses the heat produced or consumed by the reaction to determine the endpoint. This is important in biochemical titrations, such as the determination of how substrates bind to enzymes.
- Spectroscopy can be used to measure the absorption of light by the solution during the titration, if the spectrum of the reactant, titrant or product is known. The relative amounts of the product and reactant can be used to determine the endpoint. Alternately, the presence of free titrant (indicating that the reaction is complete) can be detected at very low levels.
- Amperometry can be used as a detection technique (amperometric titration). The current due to the oxidation or reduction of either the reactants or products at a working electrode will depend on the concentration of that species in solution. The endpoint can then be detected as a change in the current. This method is most useful when the excess titrant can be reduced, as in the titration of halides with Ag+. (This is handy also in that it ignores precipitates.)
Other terms
The term back titration is used when a titration is done "backwards": instead of titrating the original analyte, one adds a known excess of a standard reagent to the solution, then titrates the excess. A back titration is useful if the end point of the reverse titration is easier to identify than the end point of the normal titration. They are also useful if the reaction between the analyte and the titrant is very slow.
Particular uses
- As applied to biodiesel, titration is the act of determining the acidity of a sample of WVO by the dropwise addition of a known base to the sample while testing with pH paper for the desired neutral pH=7 reading. By knowing how much base neutralizes an amount of WVO, we discern how much base to add to the entire batch.
- Titrations in the petrochemical or food industry to define oils, fats or biodiesel and similar substances. An example procedure for all three can be found here: [1].
- Acid number: an acid-base titration with colour indicator is used to determine the free fatty acid content. See also: pH of fatty acids.
- Iodine number: a redox titration with colour indication which indicates the amount of unsaturated fatty acids.
- Saponification value: an acid-base back titration with colour indicator or potentiometric to get a hint about the average chain length of fatty acids in a fat.
See also
External links
- Science aid: Titration An informative yet simple explanation of titration aimed at teens
- Titration - apparatus, technique and calculation
- Titration freeware - simulation of any pH vs. volume curve, distribution diagrams and real data analysis


![{\displaystyle pH=pK_{a}+\log \left({\frac {[{\mbox{base}}]}{[{\mbox{acid}}]}}\right)}](https://wikimedia.org/api/rest_v1/media/math/render/svg/85e11a9235b28c2daa03f689e44fccab89e4b526)



