Organocatalysis: Difference between revisions
m add internal links |
Minestrone Soup (talk | contribs) c |
||
| Line 5: | Line 5: | ||
[[Justus von Liebig]]'s synthesis of [[oxamide]] from [[dicyan]] and water represents the ''first organocatalytic reaction'', with [[acetaldehyde]] further identified as the first discovered pure "organocatalyst", which act similarly to the then-named "ferments", now known as [[enzymes]]. <ref name="liebig_1">{{Cite journal | volume = 113 | issue = 2 | pages = 246-247 | last = Justus von Liebig | title = Ueber die Bildung des Oxamids aus Cyan | journal = Annalen der Chemie und Pharmacie | date = 1860}}</ref><ref name="Langenbeck_1"> W. Langenbeck, ''Liebigs Ann.'' '''1929''', ''469'', 16.</ref>]] |
[[Justus von Liebig]]'s synthesis of [[oxamide]] from [[dicyan]] and water represents the ''first organocatalytic reaction'', with [[acetaldehyde]] further identified as the first discovered pure "organocatalyst", which act similarly to the then-named "ferments", now known as [[enzymes]]. <ref name="liebig_1">{{Cite journal | volume = 113 | issue = 2 | pages = 246-247 | last = Justus von Liebig | title = Ueber die Bildung des Oxamids aus Cyan | journal = Annalen der Chemie und Pharmacie | date = 1860}}</ref><ref name="Langenbeck_1"> W. Langenbeck, ''Liebigs Ann.'' '''1929''', ''469'', 16.</ref>]] |
||
In [[organic chemistry]], the term '''Organocatalysis''' (a [[concatenation]] of the terms "organic" and "catalyst") refers to a form of [[catalysis]], whereby the rate of a [[chemical reaction]] is increased by an [[organic compound|organic catalyst]] referred to as an "organocatalyst" consiting of [[carbon]], [[hydrogen]], [[sulfur]] and other nonmetal elements found in organic compounds. Because of their similarity in composition and description, they are often mistaken as a [[misnomer]] for [[enzyme]]s due to their comparable effects on reaction rates |
In [[organic chemistry]], the term '''Organocatalysis''' (a [[concatenation]] of the terms "organic" and "catalyst") refers to a form of [[catalysis]], whereby the rate of a [[chemical reaction]] is increased by an [[organic compound|organic catalyst]] referred to as an "organocatalyst" consiting of [[carbon]], [[hydrogen]], [[sulfur]] and other nonmetal elements found in organic compounds. Because of their similarity in composition and description, they are often mistaken as a [[misnomer]] for [[enzyme]]s due to their comparable effects on reaction rates and forms of catalysis involved. |
||
The term "organocatalysis" was created by MacMillan in 2000 from the old and well known concept of "organic catalysis" introduced by the German chemist Wolfgang Langenbeck; "organocatalysis" is nothing more than a new name for an old methodology, but thus gives fresh impulses for intensive research in the following years. |
The term "organocatalysis" was created by MacMillan in 2000 from the old and well known concept of "organic catalysis" introduced by the German chemist Wolfgang Langenbeck; "organocatalysis" is nothing more than a new name for an old methodology, but thus gives fresh impulses for intensive research in the following years. |
||
Revision as of 21:56, 1 June 2007

In organic chemistry, the term Organocatalysis (a concatenation of the terms "organic" and "catalyst") refers to a form of catalysis, whereby the rate of a chemical reaction is increased by an organic catalyst referred to as an "organocatalyst" consiting of carbon, hydrogen, sulfur and other nonmetal elements found in organic compounds. Because of their similarity in composition and description, they are often mistaken as a misnomer for enzymes due to their comparable effects on reaction rates and forms of catalysis involved.
The term "organocatalysis" was created by MacMillan in 2000 from the old and well known concept of "organic catalysis" introduced by the German chemist Wolfgang Langenbeck; "organocatalysis" is nothing more than a new name for an old methodology, but thus gives fresh impulses for intensive research in the following years.
Organocatalysts which display secondary amine functionality can be described as performing either enamine catalysis (by forming catalytic quantities of an active enamine nucleophile) or iminium catalysis (by forming catalytic quantities of an activated iminium electrophile). This mechanism is typical for covalent organocatalysis. Covalent binding of substrate normally requires high catalyst loading (for proline-catalysis typically 20-30 mol%). Noncovalent interactions such as hydrogen-bonding facilitates low catalyst loadings (down to 0.001 mol%) making noncovalent organocata
Noncovalent organocatalysis
Thiourea derivatives
In Nature noncovalent interactions such as hydrogen bonding ("partial protonation") play a crucial role in enzyme catalysis that is characterized by selective substrate recognition (molecular recognition), substrate activation, and enormous acceleration of organic tranformations. Based on the pioneering exmaninations by Kelly, Etter, Jorgensen, Hine, Curran, Göbel, and De Mendoza (see review articles cited below) on hydrogen bonding interactions of small, metal-free compounds with electron-rich binding sites Schreiner and co-workers peformed series of theoretical and experimental systematic investigations towards the hydrogen-bonding ability of various thiourea derivatives. This purely organic compounds revealed effective acceleration of simple Diels-Alder reaction, act like weak Lewis acid catalyst, but act through explicit double hydrogen bonding instead of covalent binding known from traditional metal-ion mediated catalysis. Schreiner and co-workers identified and indroduced electron-poor thiourea derivatives as hydrogen-bonding organocatalysts. N,N'-bis[[3,5-bis(trifluormethyl)phenyl thiourea is to date the most effective achiral thiourea derivative and combines all typical structural features for double H-bonding mediated organocatalysis:
- electron-poor
- rigid structure
- non-coordinating, electron withdrawing substituents in 3,4, and/or 5 position of a phenyl ring
- the trifluoromethyl-group is the preferred substituent
Adventages of thiourea derivatives:
- no product inhibition due to weak enthalpic binding, but specific binding-“recognition“
- low catalyst-loading (down to 0.001 mol%, see 6)
- high TOF values (up to 2,000 h–1, see 8)
- simple and inexpensive synthesis
- easily to modulate and to handele, no inert atmosphere necessary
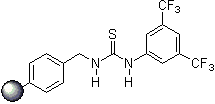
- immobilization on solid phase (polymer-bound organocatalysts), catalyst recovery and reusability
- catalysis under almost neutral conditions (pka thiourea 21.0), acid-sensitive substrates are tolerated
- metal-free, not toxic (compare traditional metal-containing Lewis-acid catalysts
- water-tolerant, even catalytically effective in water or aqueous media
- environmentally benign ("Green Chemistry")
To date various organic transformations are organocatalyzed through hydrogen-bonding N,N'-bis[[3,5-bis(trifluormethyl)phenyl thiourea at low catalyst loadings and in good to excellent product yields. This electron-poor thiourea derivative has proven to be the benchmark for noncovalent organocatalysis utilizing explicit hydrogen-bonding as well as to be the basis for development of a wide range of catalytically active derivatives.
Thiourea functionalized organocatalysts
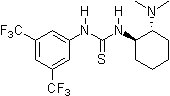
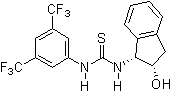
Since 2001 research groups world-wide (e.g., Berkessel, Connon, Jacobsen, Nagaswa, Takemoto) have realized the potential of thiourea derivatives and developed various achiral/chiral mono- and bifunctional derivatives incorporating the electron-poor 3,5-bis(trifluoromethyl)phenyl substrate-"anchor" functionality. Meanwhile a broad spectrum of organic transformations are performed through hydrogen-bonding organocatalysis and the research ist still in the focus of interest.
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 | |
 |
 |
References
Notes
General references on organocatalysis
- Berkessel, A., Groeger, H. (2005). Asymmetric Organocatalysis. Weinheim: Wiley-VCH. ISBN 3-527-30517-3.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Peter I. Dalko, Lionel Moisan, review: "In the Golden Age of Organocatalysis", Angew. Chem. Int. Ed. 2004, 43, 5138–5175
- Matthew J. Gaunt, Carin C.C. Johansson, Andy McNally, Ngoc T. Vo, review: "Enantioselective organocatalysis" Drug Discovery Today, 2007, 12(1/2), 8-27
- Dieter Enders, Christoph Grondal, Matthias R. M. Hüttl, review: "Asymmetric Organocatalytic Domino Reactions", Angew. Chem. Int. Ed. 2007, 46, 1570–1581
Selected references on noncovalent organocatalysis utilizing hydrogen-bonding thioureas
- Alexander Wittkopp, Peter R. Schreiner, "Diels-Alder Reactions in Water and in Hydrogen-Bonding Environments", book chapter in "The Chemistry of Dienes and Polyenes" Zvi Rappoport (Ed.), Volume 2, John Wiley & Sons Inc.; Chichester, 2000, 1029-1088. ISBN 0-471-72054-2.
- Alexander Wittkopp, "Organocatalysis of Diels-Alder Reactions by Neutral Hydrogen Bond Donors in Organic and Aqueous Solvents", dissertation written in German, Universität Göttingen, 2001. english abstract/download: [1]
- P. R. Schreiner and A. Wittkopp (2002). "H-Bonding Additives Act Like Lewis Acid Catalysts". Org. Lett. 4 (2): 217–220. doi:10.1021/ol017117s.
- A. Wittkopp and P. R. Schreiner (2003). "Metal-Free, Noncovalent Catalysis of Diels-Alder Reactions by Neutral Hydrogen Bond Donors in Organic Solvents and in Water". Chemistry - A European Journal. 9 (2): 407–414. doi:10.1002/chem.200390042.
- Peter R. Schreiner, review: "Metal-free organocatalysis through explicit hydrogen bonding interactions", Chem. Soc. Rev. 2003, 32, 289-296. abstract/download:[2]
- M. Kotke and P. R. Schreiner (2006). "Acid-free, organocatalytic acetalization". Tetrahedron. 62 (2–3): 434–439.
- Christian M. Kleiner, Peter R. Schreiner, "Hydrophobic amplification of noncovalent organocatalysis", Chem. Commun. 2006, 4315-4017.abstract/download:[3]
- M. Kotke and P. Schreiner (2007). "Generally Applicable Organocatalytic Tetrahydropyranylation of Hydroxy Functionalities with Very Low Catalyst Loading". Synthesis (5): 779–790. doi:10.1055/s-2007-965917.
- L. Wanka and C. Cabrele (2007). "γ-Aminoadamantanecarboxylic Acids Through Direct C-H Bond Amidations". European Journal of Organic Chemistry. 2007 (9): 1474–1490. doi:10.1002/ejoc.200600975.
- Z. Zhang and P. R. Schreiner (2007). "Thiourea-Catalyzed Transfer Hydrogenation of Aldimines". Synlett (9): 1455–1457. doi:10.1055/s-2007-980349.
- M. P. Petri (2004). "Activation of Carbonyl Compounds by Double Hydrogen Bonding: An Emerging Tool in Asymmetric Catalysis". Angewandte Chemie International Edition. 43 (16): 2062–2064. doi:10.1002/anie.200301732.
- Yoshiji Takemoto, review: "Recognition and activation by ureas and thioureas: stereoselective reactions using ureas and thioureas as hydrogen-bonding donors", Org. Biomol. Chem. 2005, 3, 4299-4306. abstract/download: [4]
- Mark S. Taylor, Eric N. Jacobsen (2006). "Asymmetric Catalysis by Chiral Hydrogen-Bond Donors". Angewandte Chemie International Edition. 45 (10): 1520–1543. doi:10.1002/anie.200503132.
- J. C. Stephen (2006). "Organocatalysis Mediated by (Thio)urea Derivatives". Chemistry - A European Journal. 12 (21): 5418–5427. doi:10.1002/chem.200501076.

