Reverse osmosis
| Water desalination
|
|---|
| Methods |
|
Reverse osmosis (RO) is a water purification process that uses a semi-permeable membrane to separate water molecules from other substances. RO applies pressure to overcome osmotic pressure that favors even distributions. RO can remove dissolved or suspended chemical species as well as biological substances (principally bacteria), and is used in industrial processes and the production of potable water. RO retains the solute on the pressurized side of the membrane and the purified solvent passes to the other side. The relative sizes of the various molecules determines what passes through. "Selective" membranes reject large molecules, while accepting smaller molecules (such as solvent molecules, e.g., water).[1]
RO is most commonly known for its use in drinking water purification from seawater, removing the salt and other effluent materials from the water molecules.[2]
As of 2013 the world's largest RO desalination plant was in Sorek, Israel, outputting 624 thousand cubic metres per day (165 million US gallons per day).[3]
History
A process of osmosis through semi-permeable membranes was first observed in 1748 by Jean-Antoine Nollet. For the following 200 years, osmosis was only a laboratory phenomenon. In 1950, the University of California at Los Angeles (UCLA) first investigated osmotic desalination. Researchers at both UCLA and University of Florida desalinated seawater in the mid-1950s, but the flux was too low to be commercially viable.[4] Sidney Loeb at UCLA and Srinivasa Sourirajan[5] at the National Research Council of Canada, Ottawa, found techniques for making asymmetric membranes characterized by an effectively thin "skin" layer supported atop a highly porous and much thicker substrate region. John Cadotte, of Filmtec corporation, discovered that membranes with particularly high flux and low salt passage could be made by interfacial polymerization of m-phenylene diamine and trimesoyl chloride. Cadotte's patent on this process[6] was the subject of litigation and expired. Almost all commercial RO membrane is now made by this method. By 2019, approximately 16,000 desalination plants operated around the world, producing around 95 million cubic metres per day (25 billion US gallons per day). Around half of this capacity was in the Middle East and North Africa region.[7]

In 1977 Cape Coral, Florida became the first US municipality to use RO at scale, with an initial operating capacity of 11.35 million liters (3 million US gal) per day. By 1985, rapid growth led the city to operate the world's largest low-pressure RO plant, producing 56.8 million liters (15 million US gal) per day (MGD).[8]
Osmosis
In (forward) osmosis, the solvent moves from an area of low solute concentration (high water potential), through a membrane, to an area of high solute concentration (low water potential). The driving force for the movement of the solvent is the reduction in the Gibbs free energy of the system in which the difference in solvent concentration between the sides of a membrane is reduced. This is called osmotic pressure. It reduces as the solvent moves into the more concentrated solution. Applying an external pressure to reverse the natural flow of pure solvent, thus, is reverse osmosis. The process is similar to other membrane technology applications.
RO differs from filtration in that the mechanism of fluid flow is reversed, as the solvent crosses membrane, leaving the solute behind. The predominant removal mechanism in membrane filtration is straining, or size exclusion, where the pores are 0.01 micrometers or larger, so the process can theoretically achieve perfect efficiency regardless of parameters such as the solution's pressure and concentration. RO instead involves solvent diffusion across a membrane that is either nonporous or uses nanofiltration with pores 0.001 micrometers in size. The predominant removal mechanism is from differences in solubility or diffusivity, and the process is dependent on pressure, solute concentration, and other conditions.[9]
RO requires pressure between 2–17 bar (30–250 psi) for fresh and brackish water, and 40–82 bar (600–1200 psi) for seawater. Seawater has around 27 bar (390 psi)[10] natural osmotic pressure that must be overcome.
Membrane pore sizes vary from 0.1 to 5,000 nm. Particle filtration removes particles of 1 μm or larger. Microfiltration removes particles of 50 nm or larger. Ultrafiltration removes particles of roughly 3 nm or larger. Nanofiltration removes particles of 1 nm or larger. RO is in the final category of membrane filtration, hyperfiltration, and removes particles larger than 0.1 nm.[11]
Fresh water applications

Drinking water purification
Around the world, household drinking water purification systems, including an RO step, are commonly used for improving water for drinking and cooking.
Such systems typically include these steps:
- a sediment filter to trap particles, including rust and calcium carbonate
- a second sediment filter with smaller pores
- an activated carbon filter to trap organic chemicals and chlorine, which degrades certain types of thin-film composite membrane
- an RO thin-film composite membrane
- an ultraviolet lamp for sterilizing any microbes that survive RO
- a second carbon filter to capture chemicals that survive RO
In some systems, the carbon prefilter is replaced by a cellulose triacetate (CTA) membrane. CTA is a paper by-product membrane bonded to a synthetic layer that allows contact with chlorine in the water. These require a small amount of chlorine in the water source to prevent bacteria from forming on it. The typical rejection rate for CTA membranes is 85–95%.
The cellulose triacetate membrane rots unless protected by chlorinated water, while the thin-film composite membrane breaks down in the presence of chlorine. The thin-film composite (TFC) membrane is made of synthetic material, and requires the chlorine to be removed before the water enters the membrane. To protect the TFC membrane elements from chlorine damage, carbon filters are used as pre-treatment. TFC membranes have a higher rejection rate of 95–98% and a longer life than CTA membranes.
To work effectively, the water feeding to these units should be under pressure (typically 280 kPa (40 psi) or greater).[12]
Though Portable RO Water Purifiers are commercially available and extensively used in areas lacking cleaning potable water, in Europe such processing of natural mineral water (as defined by a European directive)[13] is not allowed. In practice, a fraction of the living bacteria pass through RO through membrane imperfections or bypass the membrane entirely through leaks in seals.
Solar-powered RO
A solar-powered desalination unit produces potable water from saline water by using a photovoltaic system to supply the energy. Solar power works well for water purification in settings lacking grid electricity and can reduce operating costs and greenhouse emissions. For example, a solar-powered desalination unit designed passed tests in Australia's Northern Territory.[14]
Sunlight's intermittent nature makes output prediction difficult without an energy storage capability. However batteries or thermal energy storage systems can provide power when the sun does not.[15]
Military
Larger scale reverse osmosis water purification units (ROWPU) exist for military use. These have been adopted by the United States armed forces and the Canadian Forces. Some models are containerized, some are trailers, and some are themselves vehicles.[citation needed]
The water is treated with a polymer to initiate coagulation. Next, it is run through a multi-media filter where it undergoes primary treatment, removing turbidity. It is then pumped through a cartridge filter which is usually spiral-wound cotton. This process strips any particles larger than 5 μm and eliminates almost all turbidity.
The clarified water is then fed through a high-pressure piston pump into a series of RO vessels. 90.00–99.98% of the raw water's total dissolved solids are removed and military standards require that the result have no more than 1000–1500 parts per million by measure of electrical conductivity. It is then disinfected with chlorine.[citation needed]
Water and wastewater purification
RO-purified rainwater collected from storm drains is used for landscape irrigation and industrial cooling in Los Angeles and other cities.
In industry, RO removes minerals from boiler water at power plants.[16] The water is distilled multiple times to ensure that it does not leave deposits on the machinery or cause corrosion.
RO is used to clean effluent and brackish groundwater. The effluent in larger volumes (more than 500 m3/day) is treated in a water treatment plant first, and then the effluent runs through RO. This hybrid process reduces treatment cost significantly and lengthens membrane life.
RO can be used for the production of deionized water.[17]
In 2002, Singapore announced that a process named NEWater would be a significant part of its water plans. RO would be used to treat wastewater before discharging the effluent into reservoirs.
Food industry
Reverse osmosis is a more economical way to concentrate liquids (such as fruit juices) than conventional heat-treatment. Concentration of orange and tomato juice has advantages including a lower operating cost and the ability to avoid heat-treatment, which makes it suitable for heat-sensitive substances such as protein and enzymes.
RO is used in the dairy industry to produce whey protein powders and concentrate milk. The whey (liquid remaining after cheese manufacture) is concentrated with RO from 6% solids to 10–20% solids before ultrafiltration processing. The retentate can then be used to make whey powders, including whey protein isolate. Additionally, the permeate, which contains lactose, is concentrated by RO from 5% solids to 18–total solids to reduce crystallization and drying costs.
Although RO was once avoided in the wine industry, it is now widespread. An estimated 60 RO machines were in use in Bordeaux, France, in 2002. Known users include many of elite firms, such as Château Léoville-Las Cases.
Maple syrup production
In 1946, some maple syrup producers started using RO to remove water from sap before boiling the sap to syrup. RO allows about 75–90% of the water to be removed, reducing energy consumption and exposure of the syrup to high temperatures.
Low-alcohol beer
When beer at typical concentration is subjected to reverse osmosis, both water and alcohol pass across the membrane more readily than other components, leaving a "beer concentrate". The concentrate is then diluted with fresh water to restore the non-volatile components to their original intensity.[18]
Hydrogen production
For small-scale hydrogen production, RO is sometimes used to prevent formation of mineral deposits on the surface of electrodes.
Aquariums
Many reef aquarium keepers use RO systems to make fish-friendly seawater. Ordinary tap water can contain excessive chlorine, chloramines, copper, nitrates, nitrites, phosphates, silicates, or other chemicals detrimental to marine organisms. Contaminants such as nitrogen and phosphates can lead to unwanted algae growth. An effective combination of both RO and deionization is popular among reef aquarium keepers, and is preferred above other water purification processes due to the low cost of ownership and operating costs. Where chlorine and chloramines are found in the water, carbon filtration is needed before RO, as common residential membranes do not address these compounds.
Freshwater aquarists also use RO to duplicate the soft waters found in many tropical waters. While many tropical fish can survive in treated tap water, breeding can be impossible. Many aquatic shops sell containers of RO water for this purpose.
Window cleaning
An increasingly popular method of cleaning windows is the "water-fed pole" system. Instead of washing windows with conventional detergent, they are scrubbed with purified water, typically containing less than 10 ppm dissolved solids, using a brush on the end of a pole wielded from ground level. RO is commonly used to purify the water.
Landfill leachate purification
Treatment with RO is limited, resulting in low recoveries on high concentration (measured with electrical conductivity) and membrane fouling. RO applicability is limited by conductivity, organics, and scaling inorganic elements such as CaSO4, Si, Fe and Ba. Low organic scaling can use two different technologies: spiral wound membrane, and (for high organic scaling, high conductivity and higher pressure (up to 90 bars)), disc tube modules with RO membranes can be used. Disc tube modules were redesigned for landfill leachate purification that is usually contaminated with organic material. Due to the cross-flow, it is given a flow booster pump that recirculates the flow over the membrane between 1.5 and 3 times before it is released as a concentrate. High velocity protects against membrane scaling and allows membrane cleaning.
Power consumption for a disc tube module system

| Energy consumption per m3 leachate | |||
|---|---|---|---|
| name of module | 1-stage up to 75 bar | 2-stage up to 75 bar | 3-stage up to 120 bar |
| disc tube module | 6.1–8.1 kWh/m3 | 8.1–9.8 kWh/m3 | 11.2–14.3 kWh/m3 |
Desalination
Areas that have limited surface water or groundwater may choose to desalinate. RO is an increasingly common method, because of its relatively low energy consumption.[19]
Energy consumption is around 3 kWh/m3 (11,000 J/L), with the development of more efficient energy recovery devices and improved membrane materials. According to the International Desalination Association, for 2011, RO was used in 66% of installed desalination capacity (0.0445 of 0.0674 km3/day), and nearly all new plants.[20] Other plants use thermal distillation methods: multiple-effect distillation, and multi-stage flash.
Sea-water RO (SWRO) desalination requires around 3 kWh/m3, much higher than those required for other forms of water supply, including RO treatment of wastewater, at 0.1 to 1 kWh/m3. Up to 50% of the seawater input can be recovered as fresh water, though lower recovery rates may reduce membrane fouling and energy consumption.
Brackish water reverse osmosis (BWRO) is the desalination of water with less salt than seawater, usually from river estuaries or saline wells. The process is substantially the same as SWRO, but requires lower pressures and less energy.[1] Up to 80% of the feed water input can be recovered as fresh water, depending on feed salinity.
The Ashkelon desalination plant in Israel is the world's largest.[21][22][23]
The typical single-pass SWRO system consists of:
- Intake
- Pretreatment
- High-pressure pump (if not combined with energy recovery)
- Membrane assembly
- Energy recovery (if used)
- Remineralisation and pH adjustment
- Disinfection
- Alarm/control panel
Pretreatment
Pretreatment is important when working nanofiltration membranes due to their spiral-wound design. The material is engineered to allow one-way flow. The design does not allow for backpulsing with water or air agitation to scour its surface and remove accumulated solids. Since material cannot be removed from the membrane surface, it is susceptible to fouling (loss of production capacity). Therefore, pretreatment is a necessity for any RO or nanofiltration system. Pretreatment has four major components:
- Screening solids: Solids must be removed and the water treated to prevent membrane fouling by particle or biological growth, and reduce the risk of damage to high-pressure components.
- Cartridge filtration: String-wound polypropylene filters are typically used to remove particles of 1–5 μm diameter.
- Dosing: Oxidizing biocides, such as chlorine, are added to kill bacteria, followed by bisulfite dosing to deactivate the chlorine that can destroy a thin-film composite membrane. Biofouling inhibitors do not kill bacteria, while preventing them from growing slime on the membrane surface and plant walls.
- Prefiltration pH adjustment: If the pH, hardness and the alkalinity in the feedwater result in scaling while concentrated in the reject stream, acid is dosed to maintain carbonates in their soluble carbonic acid form.
- CO32− + H3O+ = HCO3− + H2O
- HCO3− + H3O+ = H2CO3 + H2O
- Carbonic acid cannot combine with calcium to form calcium carbonate scale. Calcium carbonate scaling tendency is estimated using the Langelier saturation index. Adding too much sulfuric acid to control carbonate scales may result in calcium sulfate, barium sulfate, or strontium sulfate scale formation on the membrane.
- Prefiltration antiscalants: Scale inhibitors (also known as antiscalants) prevent formation of more scales than acid, which can only prevent formation of calcium carbonate and calcium phosphate scales. In addition to inhibiting carbonate and phosphate scales, antiscalants inhibit sulfate and fluoride scales and disperse colloids and metal oxides. Despite claims that antiscalants can inhibit silica formation, no concrete evidence proves that silica polymerization is inhibited by antiscalants. Antiscalants can control acid-soluble scales at a fraction of the dosage required to control the same scale using sulfuric acid.[24]
- Some small-scale desalination units use 'beach wells'. These are usually drilled on the seashore. These intake facilities are relatively simple to build and the seawater they collect is pretreated via slow filtration through subsurface sand/seabed formations. Raw seawater collected using beach wells is often of better quality in terms of solids, silt, oil, grease, organic contamination, and microorganisms, compared to open seawater intakes. Beach intakes may also yield source water of lower salinity.
High pressure pump
The high pressure pump pushes water through the membrane. Typical pressures for brackish water range from 1.6 to 2.6 MPa (225 to 376 psi). In the case of seawater, they range from 5.5 to 8 MPa (800 to 1,180 psi). This requires substantial energy. Where energy recovery is used, part of the high pressure pump's work is done by the energy recovery device, reducing energy inputs.
Membrane assembly


The membrane assembly consists of a pressure vessel with a membrane that allows feedwater to be pushed against it. The membrane must be strong enough to withstand the pressure. RO membranes are made in a variety of configurations. The two most common are spiral-wound and hollow-fiber.
Only part of the water pumped onto the membrane passes through. The left-behind "concentrate" passes along the saline side of the membrane and flushes away the salt and other remnants. The percentage of desalinated water is the "recovery ratio". This varies with salinity and system design parameters: typically 20% for small seawater systems, 40% – 50% for larger seawater systems, and 80% – 85% for brackish water. The concentrate flow is typically 3 bar/50 psi less than the feed pressure, and thus retains much of the input energy.
The desalinated water purity is a function of the feed water salinity, membrane selection and recovery ratio. To achieve higher purity a second pass can be added which generally requires another pumping cycle. Purity expressed as total dissolved solids typically varies from 100 to 400 parts per million (ppm or mg/litre) on a seawater feed. A level of 500 ppm is generally the upper limit for drinking water, while the US Food and Drug Administration classifies mineral water as water containing at least 250 ppm.
Energy recovery

1: Sea water inflow,
2: Fresh water flow (40%),
3: Concentrate flow (60%),
4: Sea water flow (60%),
5: Concentrate (drain),
A: Pump flow (40%),
B: Circulation pump,
C: Osmosis unit with membrane,
D: Pressure exchanger
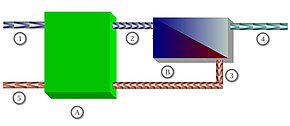
1: Sea water inflow (100%, 1 bar),
2: Sea water flow (100%, 50 bar),
3: Concentrate flow (60%, 48 bar),
4: Fresh water flow (40%, 1 bar),
5: Concentrate to drain (60%,1 bar),
A: Pressure recovery pump,
B: Osmosis unit with membrane
Energy recovery can reduce energy consumption by 50% or more. Much of the input energy can be recovered from the concentrate flow, and the increasing efficiency of energy recovery devices greatly reduces energy requirements. Devices used, in order of invention, are:
- Turbine or Pelton wheel: a water turbine driven by the concentrate flow, connected to the pump drive shaft provides part of the input power. Positive displacement axial piston motors have been used in place of turbines on smaller systems.
- Turbocharger: a water turbine driven by concentrate flow, directly connected to a centrifugal pump that boosts the output pressure, reducing the pressure needed from the pump and thereby its energy input,[25] similar in construction principle to car engine turbochargers.
- Pressure exchanger: using the pressurized concentrate flow, via direct contact or a piston, to pressurize part of the membrane feed flow to near concentrate flow pressure.[26] A boost pump then raises this pressure by typically 3 bar / 50 psi to the membrane feed pressure. This reduces flow needed from the high-pressure pump by an amount equal to the concentrate flow, typically 60%, and thereby its energy input. These are widely used on larger low-energy systems. They are capable of 3 kWh/m3 or less energy consumption.
- Energy-recovery pump: a reciprocating piston pump. The pressurized concentrate flow is applied to one side of each piston to help drive the membrane feed flow from the opposite side. These are the simplest energy recovery devices to apply, combining the high pressure pump and energy recovery in a single self-regulating unit. These are widely used on smaller low-energy systems. They are capable of 3 kWh/m3 or less energy consumption.
- Batch operation: RO systems run with a fixed volume of fluid (thermodynamically a closed system) do not suffer from wasted energy in the brine stream, as the energy to pressurize a virtually incompressible fluid (water) is negligible. Such systems have the potential to reach second-law efficiencies of 60%.[1][27][28]
Remineralisation and pH adjustment
The desalinated water is stabilized to protect downstream pipelines and storage, usually by adding lime or caustic soda to prevent corrosion of concrete-lined surfaces. Liming material is used to adjust pH between 6.8 and 8.1 to meet the potable water specifications, primarily for effective disinfection and for corrosion control. Remineralisation may be needed to replace minerals removed from the water by desalination, although this process has proved to be costly and inconvenient in order to meet mineral demand by humans and plants as found in typical freshwater. For instance water from Israel's national water carrier typically contains dissolved magnesium levels of 20 to 25 mg/liter, while water from the Ashkelon plant has no magnesium. Ashkelon water created magnesium-deficiency symptoms in crops, including tomatoes, basil, and flowers, and had to be remedied by fertilization. Israeli drinking water standards require a minimum calcium level of 20 mg/liter. Askelon's post-desalination treatment uses sulfuric acid to dissolve calcite (limestone), resulting in calcium concentrations of 40 to 46 mg/liter, lower than the 45 to 60 mg/liter found in typical Israeli fresh water.
Disinfection
Post-treatment disinfection provides secondary protection against compromised membranes and downstream problems. Disinfection by means of ultraviolet (UV) lamps (sometimes called germicidal or bactericidal) may be employed to sterilize pathogens that evade the RO process. Chlorination or chloramination (chlorine and ammonia) protects against pathogens that may have lodged in the distribution system downstream.[29]
Disadvantages
Large-scale industrial/municipal systems recover typically 75% to 80% of the feed water, or as high as 90%, because they can generate the required higher pressure.
Wastewater
Household RO units use a lot of water because they have low back pressure. Household RO water purifiers typically produce one liter of usable water and 3-25 liters of wastewater.[30] The remainder is discharged, usually into the drain. Because wastewater carries the rejected contaminants, recovering this water is not practical for household systems. Wastewater is typically delivered to house drains. A RO unit delivering 20 liters (5.3 U.S. gal) of treated water per day also discharge between 50 and 80 liters (13 and 21 U.S. gal). This led India's National Green Tribunal to propose a ban on RO water purification systems in areas where the total dissolved solids (TDS) measure in water is less than 500 mg/liter.[citation needed] In Delhi, large-scale use of household RO devices has increased the total water demand of the already water-parched National Capital Territory of India.[31]
Health
RO removes both harmful contaminants and desirable minerals. Some studies report some relation between long-term health effects and consumption of water low on calcium and magnesium, although these studies are of low quality.[32]
Waste-stream considerations
Depending upon the desired product, either the solvent or solute stream of RO will be waste. For food concentration applications, the concentrated solute stream is the product and the solvent stream is waste. For water treatment applications, the solvent stream is purified water and the solute stream is concentrated waste.[33] The solvent waste stream from food processing may be used as reclaimed water, but there may be fewer options for disposal of a concentrated waste solute stream. Ships may use marine dumping and coastal desalination plants typically use marine outfalls. Landlocked RO plants may require evaporation ponds or injection wells to avoid polluting groundwater or surface runoff.[34]
Research
Improving Current Membranes
Current RO membranes, thin-film composite (TFC) polyamide membranes, are being studied to find ways of improving their permeability. Through new imaging methods, researchers were able to make 3D models of membranes and examine how water flowed through them. They found that TFC membranes with areas of low flow significantly decreased water permeability.[35] By ensuring uniformity of the membranes and allowing water to flow continuously without slowing down, membrane permeability could be improved by 30%-40%.[36]
Electrodialysis
Research has examined integrating RO with electrodialysis to improve recovery of valuable deionized products, or to reduce concentrate volumes.
Low-pressure High-recovery (LPHR)
Another approach is low-pressure high-recovery multistage RO (LPHR). It produces concentrated brine and freshwater by cycling the output repeatedly through a relatively porous membrane at relatively low pressure. Each cycle removes additional impurities. Once the output is relatively pure, it is sent through a conventional RO membrane at conventional pressure to complete the filtration step. LPHR was found to be economically feasible, recovering more than 70% with an OPD between 58 and 65 bar and leaving no more than 350 ppm TDS from a seawater feed with 35,000 ppm TDS.
Carbon Nanotubes (CNTs)
Carbon nanotubes are meant to potentially solve the typical tradeoff between the permeability and the selectivity of RO membranes. CNTs present many ideal characteristics including: mechanical strength, electron affinity, and also exhibiting flexibility during modification. By restructuring carbon nanotubes and coating or impregnating them with other chemical compounds, scientists can manufacture these membranes to have all of the most desirable traits. The hope with CNT membranes is to find a combination of high water permeability while also decreasing the amount of neutral solutes taken out of the water. This would help decrease energy costs and the cost of remineralization after purification through the membrane.[37]
Graphene
Graphene membranes are meant to take advantage of their thinness to increase efficiency. Graphene is a singular layer of carbon atoms, so it is about 1000 times thinner than existing membranes. Graphene membranes are around 100 nm thick while current membranes are about 100 μm. Many researchers were concerned with the durability of graphene and if it would be able to handle RO pressures. New research finds that depending on the substrate (a supporting layer that does no filtration and only provides structural support), graphene membranes can withstand 57MPa of pressure which is about 10 times the typical pressures for seawater RO.[38]
Batch RO may offer increased energy efficiency, more durable equipment and higher salinity limits.
The conventional approach claimed that molecules cross the membrane individually. A research team devised a "solution-friction" theory, claiming that molecules in groups through transient pores. Characterizing that process could guide membrane development. The accepted theory is that individual water molecules diffuse through the membrane, termed the "solution-diffusion" model.[39]
See also
- Electrodeionization
- ERDLator
- Forward osmosis
- Microfiltration
- Reverse osmosis plant
- Richard Stover, pioneered the development of an energy-recovery device currently in use in most seawater reverse-osmosis desalination plants
- Silt density index
- Salinity gradient
- Milli-Q water
- Water pollution
- Water quality
References
- ^ a b c Warsinger, David M.; Tow, Emily W.; Nayar, Kishor G.; Maswadeh, Laith A.; Lienhard V, John H. (2016). "Energy efficiency of batch and semi-batch (CCRO) reverse osmosis desalination". Water Research. 106: 272–282. Bibcode:2016WatRe.106..272W. doi:10.1016/j.watres.2016.09.029. hdl:1721.1/105441. PMID 27728821.
- ^ Panagopoulos, Argyris; Haralambous, Katherine-Joanne; Loizidou, Maria (25 November 2019). "Desalination brine disposal methods and treatment technologies – A review". Science of the Total Environment. 693: 133545. Bibcode:2019ScTEn.69333545P. doi:10.1016/j.scitotenv.2019.07.351. ISSN 0048-9697. PMID 31374511. S2CID 199387639.
- ^ Wang, Brian (19 February 2015). "Next Big Future: Israel scales up Reverse Osmosis Desalination to slash costs with a fourth of the piping". nextbigfuture.com.
- ^ Glater, J. (1998). "The early history of reverse osmosis membrane development". Desalination. 117 (1–3): 297–309. Bibcode:1998Desal.117..297G. doi:10.1016/S0011-9164(98)00122-2.
- ^ Weintraub, Bob (December 2001). "Sidney Loeb, Co-Inventor of Practical Reverse Osmosis". Bulletin of the Israel Chemical Society (8): 8–9.
- ^ Cadotte, John E. (1981) "Interfacially synthesized reverse osmosis membrane" U.S. patent 4,277,344
- ^ Jones, Edward; et al. (20 March 2019). "The state of desalination and brine production: A global outlook". Science of the Total Environment. 657: 1343–1356. Bibcode:2019ScTEn.657.1343J. doi:10.1016/j.scitotenv.2018.12.076. PMID 30677901. S2CID 59250859.
- ^ 2012 Annual Consumer Report on the Quality of Tap Water Archived 4 March 2016 at the Wayback Machine. City of Cape Coral
- ^ Crittenden, John; Trussell, Rhodes; Hand, David; Howe, Kerry and Tchobanoglous, George (2005). Water Treatment Principles and Design, 2nd ed. John Wiley and Sons. New Jersey. ISBN 0-471-11018-3
- ^ Lachish, Uri. "Optimizing the Efficiency of Reverse Osmosis Seawater Desalination". guma science.
- ^ "Purification of Contaminated Water with Reverse Osmosis" ISSN 2250-2459, ISO 9001:2008 Certified Journal, Volume 3, Issue 12, December 2013
- ^ Knorr, Erik Voigt, Henry Jaeger, Dietrich (2012). Securing Safe Water Supplies : comparison of applicable technologies (Online-Ausg. ed.). Oxford: Academic Press. p. 33. ISBN 978-0124058866.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Council Directive of 15 July 1980 on the approximation of the laws of the Member States relating to the exploitation and marketing of natural mineral waters. eur-lex.europa.eu
- ^ "Award-winning Solar Powered Desalination Unit aims to solve Central Australian water problems". University of Wollongong. 4 November 2005. Retrieved 19 July 2017.
- ^ Low temperature desalination using solar collectors augmented by thermal energy storage
- ^ Shah, Vishal, ed. (2008). Emerging Environmental Technologies. Dordrecht: Springer Science. p. 108. ISBN 978-1402087868.
- ^ Grabowski, Andrej (2010). Electromembrane desalination processes for production of low conductivity water. Berlin: Logos-Verl. ISBN 978-3832527143.
- ^ Lewis, Michael J; Young, Tom W (6 December 2012). Brewing (2 ed.). New York: Kluwer. p. 110. ISBN 978-1-4615-0729-1.
- ^ Warsinger, David M.; Mistry, Karan H.; Nayar, Kishor G.; Chung, Hyung Won; Lienhard V, John H. (2015). "Entropy Generation of Desalination Powered by Variable Temperature Waste Heat". Entropy. 17 (11): 7530–7566. Bibcode:2015Entrp..17.7530W. doi:10.3390/e17117530. hdl:1721.1/100423.
- ^ International Desalination Association Yearbook 2012–13
- ^ Israel is No. 5 on Top 10 Cleantech List in Israel 21c A Focus Beyond Archived 16 October 2010 at the Wayback Machine Retrieved 21 December 2009
- ^ Desalination Plant Seawater Reverse Osmosis (SWRO) Plant. Water-technology.net
- ^ Sauvetgoichon, B (2007). "Ashkelon desalination plant — A successful challenge". Desalination. 203 (1–3): 75–81. Bibcode:2007Desal.203...75S. doi:10.1016/j.desal.2006.03.525.
- ^ Malki, M. (2008). "Optimizing scale inhibition costs in reverse osmosis desalination plants". International Desalination and Water Reuse Quarterly. 17 (4): 28–29.
- ^ Yu, Yi-Hsiang; Jenne, Dale (8 November 2018). "Numerical Modeling and Dynamic Analysis of a Wave-Powered Reverse-Osmosis System". Journal of Marine Science and Engineering. 6 (4). MDPI AG: 132. doi:10.3390/jmse6040132. ISSN 2077-1312.
- ^ Stover, Richard L. (2007). "Seawater reverse osmosis with isobaric energy recovery devices". Desalination. 203 (1–3). Elsevier BV: 168–175. Bibcode:2007Desal.203..168S. doi:10.1016/j.desal.2006.03.528. ISSN 0011-9164.
- ^ Cordoba, Sandra; Das, Abhimanyu; Leon, Jorge; Garcia, Jose M; Warsinger, David M (2021). "Double-acting batch reverse osmosis configuration for best-in-class efficiency and low downtime". Desalination. 506. Elsevier BV: 114959. Bibcode:2021Desal.50614959C. doi:10.1016/j.desal.2021.114959. ISSN 0011-9164. S2CID 233553757.
- ^ Wei, Quantum J.; Tucker, Carson I.; Wu, Priscilla J.; Trueworthy, Ali M.; Tow, Emily W.; Lienhard, John H. (2020). "Impact of salt retention on true batch reverse osmosis energy consumption: Experiments and model validation". Desalination. 479. Elsevier BV: 114177. Bibcode:2020Desal.47914177W. doi:10.1016/j.desal.2019.114177. hdl:1721.1/124221. ISSN 0011-9164. S2CID 213654912.
- ^ Sekar, Chandru. "IEEE R10 HTA Portable Autonomous Water Purification System". IEEE. Retrieved 4 March 2015.
- ^ "Learn The Pros And Cons Of Reverse Osmosis Water Filtration Systems". Forbes. 26 April 2022. Retrieved 8 October 2023.
- ^ Singh, Govind (2017). "Implication of Household Use of R.O. Devices for Delhi's Urban Water Scenario". Journal of Innovation for Inclusive Development. 2 (1): 24–29. Archived from the original on 17 May 2017. Retrieved 15 April 2017.
- ^ Kozisek, Frantisek. "Health risks from drinking demineralised water" (PDF). Czech Republic: National Institute of Public Health. Archived from the original (PDF) on 7 February 2022.
- ^ Weber, Walter J. (1972). Physicochemical Processes for Water Quality Control. New York: John Wiley & Sons. p. 320. ISBN 9780471924357. OCLC 1086963937.
- ^ Hammer, Mark J. (1975). Water and Waste-Water Technology. New York: John Wiley & Sons. p. 266. ISBN 9780471347262.
- ^ Culp, Tyler E.; Khara, Biswajit; Brickey, Kaitlyn P.; Geitner, Michael; Zimudzi, Tawanda J.; Wilbur, Jeffrey D.; Jons, Steven D.; Roy, Abhishek; Paul, Mou; Ganapathysubramanian, Baskar; Zydney, Andrew L.; Kumar, Manish; Gomez, Enrique D. (January 2021). "Nanoscale control of internal inhomogeneity enhances water transport in desalination membranes". Science. 371 (6524): 72–75. Bibcode:2021Sci...371...72C. doi:10.1126/science.abb8518. ISSN 0036-8075. PMID 33384374. S2CID 229935140.
- ^ "Desalination breakthrough could lead to cheaper water filtration". ScienceDaily. Retrieved 26 May 2023.
- ^ Ali, Sharafat; Rehman, Syed Aziz Ur; Luan, Hong-Yan; Farid, Muhammad Usman; Huang, Haiou (1 January 2019). "Challenges and opportunities in functional carbon nanotubes for membrane-based water treatment and desalination". Science of the Total Environment. 646: 1126–1139. Bibcode:2019ScTEn.646.1126A. doi:10.1016/j.scitotenv.2018.07.348. ISSN 0048-9697. PMID 30235599. S2CID 52311560.
- ^ Cohen-Tanugi, David; Grossman, Jeffrey C. (12 November 2014). "Mechanical Strength of Nanoporous Graphene as a Desalination Membrane". Nano Letters. 14 (11): 6171–6178. Bibcode:2014NanoL..14.6171C. doi:10.1021/nl502399y. ISSN 1530-6984. PMID 25357231.
- ^ Levy, Max G. "Everyone Was Wrong About Reverse Osmosis—Until Now". Wired. ISSN 1059-1028. Retrieved 20 May 2023.
Sources
- Metcalf; Eddy (1972). Wastewater Engineering. New York: McGraw-Hill Book Company. ISBN 978-0-070-49539-5.

