Patatin

Patatin is a family of glycoproteins found in potatoes (Solanum tuberosum) and is also known as tuberin as it is commonly found within vacuoles of parenchyma tissue in the tuber of the plant. They consist of about 366 amino acids all making up and isoelectric point of 4.9. They have a molecular weight ranging from 40 to 45 kDa, but are commonly found as a 80kDa dimer.[1] The main function of patatin is as a storage protein but it also has lipase activity and can cleave fatty acids from membrane lipids.[2][3] The patatin protein makes up about 40% of the soluble protein in potato tubers.[4] Members of this protein family have also been found in animals.
Allergy
Patatin is identified as a major cause of potato allergy.[5] It has found to be similar to latex, and when in contact with open skin, there has been an increase of immunoglobulin E which causes some allergic reactions and symptoms, such as asthmatic symptoms, or atopic dermatitis.[6] It is unclear as to why the plant does this, however it could be a potential defense mechanism against insects.[7]
Function
Functionally, patatin serves as a key contributor to the antioxidant activity in potato tubers, in order to keep the potato fresh. Additionally, they function as acyl hydrolases, which breaks down different types of substrates. Notably, patatins also demonstrate β-1,3-glucanase activity, suggesting their involvement in breaking down polysaccharides. This diverse enzymatic activity contributes to ensuring the nutritional composition of the potato. Beyond their role as storage proteins, patatins play a significant part in the plant's defense mechanisms against pests and fungal pathogens. The galactolipase and β-1,3-glucanase activities exhibited by patatins are believed to contribute to the plant's resistance to external threats. This dual functionality underscores the importance of patatins in safeguarding the potato plant against potential environmental challenges.[8]
Beyond its role as a storage protein, patatin's functions extend to antioxidant activity and categorization as an esterase enzyme complex. It demonstrates enzymatic activity in lipid metabolism through lipid acyl hydrolases (LAHs) and acyl transferases. This activity varies across potato cultivars, extraction techniques, and fatty acid substrates.[9]
Structure
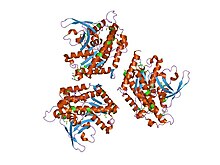
The patatin genes are located at a single major locus, comprising both functional and non-functional genes. Patatin isoforms exhibit considerable variability among different potato cultivars. Patatin's primary residence in the vacuole, alongside protease inhibitor variants, positions it as a major player in potato tuber proteins. The ngLOC software predicts 296 vacuolar proteins, with 450 putative vacuolar proteins identified through mass spectrometric sequencing. Notably, the tuber vacuole is recognized as a protein storage vacuole, with a distinct absence of proteolytic or glycolytic enzymes. Structurally, patatin emerges as a tertiary stabilized protein, exhibiting stability up to 45 °C. Beyond this threshold, its secondary structure begins to unfold, with the α-helical portion denaturing at 55 °C. This vulnerability to temperature changes highlights the delicate balance in maintaining its structural integrity.[10]
Patatin's hydrolase activity, attributed to its parallel β-sheet core with a catalytic serine located in the nucleophilic elbow loop, places it within the hydrolase family. This core structure is crucial for its lipid acyl hydrolase (LAH) activity, providing insights into its enzymatic functions and potential participation in plant defenses.[11]
There is a study delves into the multifaceted properties of patatin, the predominant protein in potatoes, revealing its structural diversity through the identification of several isoforms. Notably rich in essential amino acids, patatin emerges as a valuable source of nutrition. The glycoprotein nature of patatin, characterized by O-linked glycosylation, incorporates various monosaccharides, including fucose, indicating a fucosylated glycan structural feature. The specific binding of patatin to AAL, a fucose-affine lectin, underscores its distinctive glycan composition. Moving beyond its molecular characteristics, the research explores the regulatory effects of patatin on lipid metabolism, fat catabolism, fat absorption, and lipase activity in zebrafish larvae subjected to high-fat feeding. Results suggest that patatin, at a concentration of 37.0 μg/mL, promotes lipid decomposition metabolism by 23% and exhibits inhibitory effects on lipase activity and fat absorption, positioning it as a potential natural constituent with anti-obesity properties. These findings illuminate the diverse facets of patatin, shedding light on its nutritional significance and its prospective role in combating obesity.[12]
Isoforms
Patatin is a complex assembly of proteins represented by two multigene families: class I in large concentrations in the tuber and class II in smaller concentrations throughout the potato plant. Isoforms A, B, C, and D exhibit charge-based differences, with isoform A presenting the lowest surface charge. These isoforms, homologous in nature, differ in molecular masses and ratios, showcasing their structural diversity.
Glycosylation
Patatin isoforms undergo glycosylation, impacting their molecular masses and contributing to variations between isoforms. Experimental discrepancies in molar mass differences indicate potential glycosylation between protein and carbohydrates in potatoes. This glycosylation may play a role in the protein's functional characteristics.[11]
Patatin-like phospholipase
The patatin-like phospholipase (PNPLA) domain, found in proteins encoding patatin, is widespread across diverse life forms, spanning eukaryotes and prokaryotes. These proteins are involved in a variety of biological functions, encompassing sepsis induction, host colonization, triglyceride metabolism, and membrane trafficking. Key features of PNPLA domain-containing proteins include their lipase and transacylase properties, signifying their significant roles in maintaining lipid and energy homeostasis across different organisms and biological contexts.[13]
References
- ^ Racusen D, Weller DL (1984). "Molecular Weight of Patatin, a Major Potato Tuber Protein1". Journal of Food Biochemistry. 8 (2): 103–107. doi:10.1111/j.1745-4514.1984.tb00318.x. ISSN 1745-4514.
- ^ Shewry PR (June 2003). "Tuber storage proteins". Annals of Botany. 91 (7): 755–769. doi:10.1093/aob/mcg084. PMC 4242388. PMID 12730067.
- ^ Bánfalvi Z, Kostyál Z, Barta E (November 1994). "Solanum brevidens possesses a non-sucrose-inducible patatin gene". Molecular & General Genetics. 245 (4): 517–522. doi:10.1007/BF00302265. PMID 7808402. S2CID 6862380.
- ^ Mignery GA, Pikaard CS, Park WD (1988). "Molecular characterization of the patatin multigene family of potato". Gene. 62 (1): 27–44. doi:10.1016/0378-1119(88)90577-X. PMID 3371664.
- ^ Chipps BE (5 March 2000). "Food Allergy: New Insights and Management Strategies". Potato Allergy. Medscape.
- ^ Seppälä U, Alenius H, Turjanmaa K, Reunala T, Palosuo T, Kalkkinen N (January 1999). "Identification of patatin as a novel allergen for children with positive skin prick test responses to raw potato". The Journal of Allergy and Clinical Immunology. 103 (1 Pt 1): 165–171. doi:10.1016/s0091-6749(99)70541-5. PMID 9893201.
- ^ Srivastava LM (2002). "Vegetative storage protein, tuberization, senescence, and abscission. 4. Defense related proteins.". Plant growth and development. Hormones and environment. San Diego: Academic Press. pp. 473–520. doi:10.1016/B978-012660570-9/50162-3.
- ^ Waglay A, Karboune S (January 2016). "Chapter 4 - Potato Proteins: Functional Food Ingredients". In Singh J, Kaur L (eds.). Advances in Potato Chemistry and Technology (Second ed.). San Diego: Academic Press. pp. 75–104. doi:10.1016/B978-0-12-800002-1.00004-2. ISBN 978-0-12-800002-1.
- ^ Kärenlampi SO, White PJ (January 2009). "Chapter 5 - Potato Proteins, Lipids, and Minerals". In Singh J, Kaur L (eds.). Advances in Potato Chemistry and Technology. San Diego: Academic Press. pp. 99–125. doi:10.1016/B978-0-12-374349-7.00005-2. ISBN 978-0-12-374349-7.
- ^ González-Pérez S, Arellano JB (2009-01-01). "15 - Vegetable protein isolates". In Phillips GO, Williams PA (eds.). Handbook of Hydrocolloids. Woodhead Publishing Series in Food Science, Technology and Nutrition (Second ed.). Woodhead Publishing. pp. 383–419. doi:10.1533/9781845695873.383. hdl:10261/21904. ISBN 978-1-84569-414-2.
- ^ a b Alting AC, Pouvreau L, Giuseppin ML, van Nieuwenhuijzen NH (January 2011). "12 - Potato proteins". In Phillips GO, Williams PA (eds.). Handbook of Food Proteins. Woodhead Publishing Series in Food Science, Technology and Nutrition. Woodhead Publishing. pp. 316–334. doi:10.1533/9780857093639.316. ISBN 978-1-84569-758-7.
- ^ Wu J, Wu Q, Yang D, Zhou M, Xu J, Wen Q, et al. (May 2021). "Patatin primary structural properties and effects on lipid metabolism". Food Chemistry. 344: 128661. doi:10.1016/j.foodchem.2020.128661. PMID 33272761. S2CID 227282218.
- ^ Liu G, Zhang K, Ai J, Deng X, Hong Y, Wang X (November 2015). "Patatin-related phospholipase A, pPLAIIIα, modulates the longitudinal growth of vegetative tissues and seeds in rice". Journal of Experimental Botany. 66 (21): 6945–55. doi:10.1093/jxb/erv402. PMC 4623698. PMID 26290597.
