Particulate inorganic carbon

Particulate inorganic carbon (PIC) can be contrasted with dissolved inorganic carbon (DIC), the other form of inorganic carbon found in the ocean. These distinctions are important in chemical oceanography. Particulate inorganic carbon is sometimes called suspended inorganic carbon. In operational terms, it is defined as the inorganic carbon in particulate form that is too large to pass through the filter used to separate dissolved inorganic carbon.
Most PIC is calcium carbonate, CaCO3, particularly in the form of calcite, but also in the form of aragonite. Calcium carbonate makes up the shells of many marine organisms. It also forms during whiting events and is excreted by marine fish during osmoregulation.
Overview
| Part of a series on the |
| Carbon cycle |
|---|
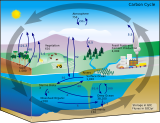 |
Carbon compounds can be distinguished as either organic or inorganic, and dissolved or particulate, depending on their composition. Organic carbon forms the backbone of key component of organic compounds such as – proteins, lipids, carbohydrates, and nucleic acids. Inorganic carbon is found primarily in simple compounds such as carbon dioxide, carbonic acid, bicarbonate, and carbonate (CO2, H2CO3, HCO3−, CO32− respectively).
Marine carbon is further separated into particulate and dissolved phases. These pools are operationally defined by physical separation – dissolved carbon passes through a 0.2 μm filter, and particulate carbon does not.
There are two main types of inorganic carbon that are found in the oceans. Dissolved inorganic carbon (DIC) is made up of bicarbonate (HCO3−), carbonate (CO32−) and carbon dioxide (including both dissolved CO2 and carbonic acid H2CO3). DIC can be converted to particulate inorganic carbon (PIC) through precipitation of CaCO3 (biologically or abiotically). DIC can also be converted to particulate organic carbon (POC) through photosynthesis and chemoautotrophy (i.e. primary production). DIC increases with depth as organic carbon particles sink and are respired. Free oxygen decreases as DIC increases because oxygen is consumed during aerobic respiration.
Particulate inorganic carbon (PIC) is the other form of inorganic carbon found in the ocean. Most PIC is the CaCO3 that makes up shells of various marine organisms, but can also form in whiting events. Marine fish also excrete calcium carbonate during osmoregulation.[4]
Some of the inorganic carbon species in the ocean, such as bicarbonate and carbonate, are major contributors to alkalinity, a natural ocean buffer that prevents drastic changes in acidity (or pH). The marine carbon cycle also affects the reaction and dissolution rates of some chemical compounds, regulates the amount of carbon dioxide in the atmosphere and Earth's temperature.[5]


Calcium carbonate
Particulate inorganic carbon (PIC) usually takes the form of calcium carbonate (CaCO3), and plays a key part in the ocean carbon cycle.[8] This biologically fixed carbon is used as a protective coating for many planktonic species (coccolithophores, foraminifera) as well as larger marine organisms (mollusk shells). Calcium carbonate is also excreted at high rates during osmoregulation by fish, and can form in whiting events.[9] While this form of carbon is not directly taken from the atmospheric budget, it is formed from dissolved forms of carbonate which are in equilibrium with CO2 and then responsible for removing this carbon via sequestration.[10]
- CO2 + H2O → H2CO3 → H+ + HCO3−
- Ca2+ + 2HCO3− → CaCO3 + CO2 + H2O
While this process does manage to fix a large amount of carbon, two units of alkalinity are sequestered for every unit of sequestered carbon.[11][12] The formation and sinking of CaCO3 therefore drives a surface to deep alkalinity gradient which serves to raise the pH of surface waters, shifting the speciation of dissolved carbon to raise the partial pressure of dissolved CO2 in surface waters, which actually raises atmospheric levels. In addition, the burial of CaCO3 in sediments serves to lower overall oceanic alkalinity, tending to raise pH and thereby atmospheric CO2 levels if not counterbalanced by the new input of alkalinity from weathering.[13] The portion of carbon that is permanently buried at the sea floor becomes part of the geologic record. Calcium carbonate often forms remarkable deposits that can then be raised onto land through tectonic motion as in the case with the White Cliffs of Dover in Southern England. These cliffs are made almost entirely of the plates of buried coccolithophores.[14]
Carbonate pump

The carbonate pump, sometimes called the carbonate counter pump, starts with marine organisms at the ocean's surface producing particulate inorganic carbon (PIC) in the form of calcium carbonate (calcite or aragonite, CaCO3). This CaCO3 is what forms hard body parts like shells.[5] The formation of these shells increases atmospheric CO2 due to the production of CaCO3[15] in the following reaction with simplified stoichiometry:[16]
| Ca+2 + 2 HCO−3 ⇌ CaCO3 + CO2 + H2O[17] | (4) |
Coccolithophores, a nearly ubiquitous group of phytoplankton that produce shells of calcium carbonate, are the dominant contributors to the carbonate pump.[5] Due to their abundance, coccolithophores have significant implications on carbonate chemistry, in the surface waters they inhabit and in the ocean below: they provide a large mechanism for the downward transport of CaCO3.[18] The air-sea CO2 flux induced by a marine biological community can be determined by the rain ratio - the proportion of carbon from calcium carbonate compared to that from organic carbon in particulate matter sinking to the ocean floor, (PIC/POC).[17] The carbonate pump acts as a negative feedback on CO2 taken into the ocean by the solubility pump. It occurs with lesser magnitude than the solubility pump.
The carbonate pump is sometimes referred to as the "hard tissue" component of the biological pump.[19] Some surface marine organisms, like coccolithophores, produce hard structures out of calcium carbonate, a form of particulate inorganic carbon, by fixing bicarbonate.[20] This fixation of DIC is an important part of the oceanic carbon cycle.
- Ca2+ + 2 HCO3− → CaCO3 + CO2 + H2O
While the biological carbon pump fixes inorganic carbon (CO2) into particulate organic carbon in the form of sugar (C6H12O6), the carbonate pump fixes inorganic bicarbonate and causes a net release of CO2.[20] In this way, the carbonate pump could be termed the carbonate counter pump. It works counter to the biological pump by counteracting the CO2 flux from the biological pump.[15]
Calcite and aragonite seas

An aragonite sea contains aragonite and high-magnesium calcite as the primary inorganic calcium carbonate precipitates. The chemical conditions of the seawater must be notably high in magnesium content relative to calcium (high Mg/Ca ratio) for an aragonite sea to form. This is in contrast to a calcite sea in which seawater low in magnesium content relative to calcium (low Mg/Ca ratio) favors the formation of low-magnesium calcite as the primary inorganic marine calcium carbonate precipitate.
The Early Paleozoic and the Middle to Late Mesozoic oceans were predominantly calcite seas, whereas the Middle Paleozoic through the Early Mesozoic and the Cenozoic (including today) are characterized by aragonite seas.[21][22][23][24][25][26][27][28]
Aragonite seas occur due to several factors, the most obvious of these is a high seawater Mg/Ca ratio (Mg/Ca > 2), which occurs during intervals of slow seafloor spreading.[24] However, the sea level, temperature, and calcium carbonate saturation state of the surrounding system also determine which polymorph of calcium carbonate (aragonite, low-magnesium calcite, high-magnesium calcite) will form.[29][30]
Likewise, the occurrence of calcite seas is controlled by the same suite of factors controlling aragonite seas, with the most obvious being a low seawater Mg/Ca ratio (Mg/Ca < 2), which occurs during intervals of rapid seafloor spreading.[24][28]
Whiting events

A whiting event is a phenomenon that occurs when a suspended cloud of fine-grained calcium carbonate precipitates in water bodies, typically during summer months, as a result of photosynthetic microbiological activity or sediment disturbance.[31][32][33] The phenomenon gets its name from the white, chalky color it imbues to the water. These events have been shown to occur in temperate waters as well as tropical ones, and they can span for hundreds of meters.[33] They can also occur in both marine and freshwater environments.[34] The origin of whiting events is debated among the scientific community, and it is unclear if there is a single, specific cause. Generally, they are thought to result from either bottom sediment re-suspension or by increased activity of certain microscopic life such as phytoplankton.[35][36][31] Because whiting events affect aquatic chemistry, physical properties, and carbon cycling, studying the mechanisms behind them holds scientific relevance in various ways.[37][32][38][39][40]
Great Calcite Belt
| Part of a series on |
| Plankton |
|---|
 |
The Great Calcite Belt (GCB) of the Southern Ocean is a region of elevated summertime upper ocean calcite concentration derived from coccolithophores, despite the region being known for its diatom predominance. The overlap of two major phytoplankton groups, coccolithophores and diatoms, in the dynamic frontal systems characteristic of this region provides an ideal setting to study environmental influences on the distribution of different species within these taxonomic groups.[41]
The Great Calcite Belt, defined as an elevated particulate inorganic carbon (PIC) feature occurring alongside seasonally elevated chlorophyll a in austral spring and summer in the Southern Ocean,[42] plays an important role in climate fluctuations,[43][44] accounting for over 60% of the Southern Ocean area (30–60° S).[45] The region between 30° and 50° S has the highest uptake of anthropogenic carbon dioxide (CO2) alongside the North Atlantic and North Pacific oceans.[46] Knowledge of the impact of interacting environmental influences on phytoplankton distribution in the Southern Ocean is limited. For example, more understanding is needed of how light and iron availability or temperature and pH interact to control phytoplankton biogeography.[47][48][49] Hence, if model parameterizations are to improve to provide accurate predictions of biogeochemical change, a multivariate understanding of the full suite of environmental drivers is required.[50][41]
The Southern Ocean has often been considered as a microplankton-dominated (20–200 μm) system with phytoplankton blooms dominated by large diatoms and Phaeocystis sp.[51][52][53] However, since the identification of the GCB as a consistent feature[42][54] and the recognition of picoplankton (< 2 μm) and nanoplankton (2–20 μm) importance in high-nutrient, low-chlorophyll (HNLC) waters,[55] the dynamics of small (bio)mineralizing plankton and their export need to be acknowledged. The two dominant biomineralizing phytoplankton groups in the GCB are coccolithophores and diatoms. Coccolithophores are generally found north of the polar front,[56] though Emiliania huxleyi has been observed as far south as 58° S in the Scotia Sea,[57] at 61° S across Drake Passage,[49] and at 65°S south of Australia.[58][41]
Diatoms are present throughout the GCB, with the polar front marking a strong divide between different size fractions.[59] North of the polar front, small diatom species, such as Pseudo-nitzschia spp. and Thalassiosira spp., tend to dominate numerically, whereas large diatoms with higher silicic acid requirements (e.g., Fragilariopsis kerguelensis) are generally more abundant south of the polar front.[59] High abundances of nanoplankton (coccolithophores, small diatoms, chrysophytes) have also been observed on the Patagonian Shelf [52] and in the Scotia Sea.[60] Currently, few studies incorporate small biomineralizing phytoplankton to species level.[59][51][52][60] Rather, the focus has often been on the larger and noncalcifying species in the Southern Ocean due to sample preservation issues (i.e., acidified Lugol’s solution dissolves calcite, and light microscopy restricts accurate identification to cells > 10 μm.[60] In the context of climate change and future ecosystem function, the distribution of biomineralizing phytoplankton is important to define when considering phytoplankton interactions with carbonate chemistry,[61][62] and ocean biogeochemistry.[63][64][65][41]
The Great Calcite Belt spans the major Southern Ocean circumpolar fronts: the Subantarctic front, the polar front, the Southern Antarctic Circumpolar Current front, and occasionally the southern boundary of the Antarctic Circumpolar Current.[66][67][68] The subtropical front (at approximately 10 °C) acts as the northern boundary of the GCB and is associated with a sharp increase in PIC southwards.[45] These fronts divide distinct environmental and biogeochemical zones, making the GCB an ideal study area to examine controls on phytoplankton communities in the open ocean.[53][47] A high PIC concentration observed in the GCB (1 μmol PIC L−1) compared to the global average (0.2 μmol PIC L−1) and significant quantities of detached E. huxleyi coccoliths (in concentrations > 20,000 coccoliths mL−1)[45] both characterize the GCB. The GCB is clearly observed in satellite imagery [42] spanning from the Patagonian Shelf [69][70] across the Atlantic, Indian, and Pacific oceans and completing Antarctic circumnavigation via the Drake Passage.[41]


Coccolithophores

Since the industrial revolution 30% of the anthropogenic CO2 has been absorbed by the oceans,[71] resulting in ocean acidification,[72] which is a threat to calcifying alga.[73][74] As a result, there has been profound interest in these calcifying algae, boosted by their major role in the global carbon cycle.[75][76][77][78][79] Globally, coccolithophores, particularly Emiliania huxleyi, are considered to be the most dominant calcifying algae, which blooms can even be seen from outer space.[80] Calcifying algae create an exoskeleton from calcium carbonate platelets (coccoliths), providing ballast which enhances the organic and inorganic carbon flux to the deep sea.[75][81] Organic carbon is formed by means of photosynthesis, where CO2 is fixed and converted into organic molecules, causing removal of CO2 from the seawater. Counterintuitively, the production of coccoliths leads to the release of CO2 in the seawater, due to removal of carbonate from the seawater, which reduces the alkalinity and causes acidification.[82] Therefore, the ratio between particulate inorganic carbon (PIC) and particulate organic carbon (POC) is an important measure for the net release or uptake of CO2. In short, the PIC:POC ratio is a key characteristic required to understand and predict the impact of climate change on the global ocean carbon cycle.[83][72][77][84][85][79][86]
Calcium particle morphologies

B) and D) Particles similar to the Ca carbonates described to precipitate on the cell surface of cultured marine bacteria.
E) and F) Particles with one flat surface suggesting that they are formed on a surface or interface.
G and H) Particles with rhombohedral shape.
I) and J) Baton like particles resembling Bahaman ooids.


See also
- carbonate compensation depth
- aragonite compensation depth
- lysocline
- calcareous ooze
- Carbonate pump
- Marine biogenic calcification
- snowline: the depth at which carbonate disappear from sediments under steady-state conditions
References
- ^ Particulate Inorganic Carbon (PIC) Ocean Biology Processing Group, NASA. Accessed 24 October 2020.
- ^ Balch, W. M.; Gordon, Howard R.; Bowler, B. C.; Drapeau, D. T.; Booth, E. S. (2005). "Calcium carbonate measurements in the surface global ocean based on Moderate-Resolution Imaging Spectroradiometer data". Journal of Geophysical Research. 110 (C7): C07001. Bibcode:2005JGRC..110.7001B. doi:10.1029/2004jc002560.
- ^ Gordon, Howard R.; Boynton, G. Chris; Balch, William M.; Groom, Stephen B.; Harbour, Derek S.; Smyth, Tim J. (2001). "Retrieval of coccolithophore calcite concentration from SeaWiFS Imagery". Geophysical Research Letters. 28 (8): 1587–1590. Bibcode:2001GeoRL..28.1587G. doi:10.1029/2000gl012025. S2CID 129177844.
- ^ Wilson, R. W.; Millero, F. J.; Taylor, J. R.; Walsh, P. J.; Christensen, V.; Jennings, S.; Grosell, M. (16 January 2009). "Contribution of Fish to the Marine Inorganic Carbon Cycle". Science. 323 (5912): 359–362. Bibcode:2009Sci...323..359W. doi:10.1126/science.1157972. ISSN 0036-8075. PMID 19150840. S2CID 36321414.
- ^ a b c Emerson, Steven (2008). Chemical Oceanography and the Marine Carbon Cycle. United Kingdom: Cambridge University Press. ISBN 978-0-521-83313-4.
- ^ Davies, Emlyn J.; Basedow, Sünnje L.; McKee, David (2021). "The hidden influence of large particles on ocean colour". Scientific Reports. 11 (1): 3999. Bibcode:2021NatSR..11.3999D. doi:10.1038/s41598-021-83610-5. PMC 7889869. PMID 33597642.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Capelle, David W.; Kuzyk, Zou Zou A.; Papakyriakou, Tim; Guéguen, Céline; Miller, Lisa A.; MacDonald, Robie W. (2020). "Effect of terrestrial organic matter on ocean acidification and CO2 flux in an Arctic shelf sea". Progress in Oceanography. 185: 102319. Bibcode:2020PrOce.18502319C. doi:10.1016/j.pocean.2020.102319. hdl:1993/34767.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Mitchell, C.; Hu, C.; Bowler, B.; Drapeau, D.; Balch, W. M. (2017). "Estimating Particulate Inorganic Carbon Concentrations of the Global Ocean from Ocean Color Measurements Using a Reflectance Difference Approach". Journal of Geophysical Research: Oceans. 122 (11): 8707–8720. Bibcode:2017JGRC..122.8707M. doi:10.1002/2017JC013146.
- ^ Wilson, R. W.; Millero, F. J.; Taylor, J. R.; Walsh, P. J.; Christensen, V.; Jennings, S.; Grosell, M. (16 January 2009). "Contribution of Fish to the Marine Inorganic Carbon Cycle". Science. 323 (5912): 359–362. Bibcode:2009Sci...323..359W. doi:10.1126/science.1157972. PMID 19150840. S2CID 36321414.
- ^ Pilson MEQ. 2012. An Introduction to the Chemistry of the Sea. Cambridge University Press, pp.
- ^ Hain, M.P.; Sigman, D.M.; Haug, G.H. (2014). The Biological Pump in the Past (PDF). Vol. 8. pp. 485–517. doi:10.1016/B978-0-08-095975-7.00618-5. ISBN 9780080983004. Archived from the original (PDF) on 11 February 2018. Retrieved 1 June 2015.
{{cite book}}:|journal=ignored (help) - ^ Hain, M.P.; Sigman, D.M.; Haug, G.H. (2010). "Carbon dioxide effects of Antarctic stratification, North Atlantic Intermediate Water formation, and subantarctic nutrient drawdown during the last ice age: Diagnosis and synthesis in a geochemical box model". Global Biogeochemical Cycles. 24 (4): 1–19. Bibcode:2010GBioC..24.4023H. doi:10.1029/2010GB003790.
- ^ Sigman DM & GH Haug. 2006. The biological pump in the past. In: Treatise on Geochemistry; vol. 6, (ed.). Pergamon Press, pp. 491-528
- ^ Webb, Paul (2019) Introduction to Oceanography, Chapter 12: Ocean Sediments, page 273–297, Rebus Community. Updated 2020.
- ^ a b Zeebe, R.E., 2016. "The calcium carbonate counter pump: Fundamentals, evolution through time, and future feedbacks". American Geophysical Union, pp.B23A-08.
- ^ "ASLO : Limnology & Oceanography: e-Books". aslo.org. Archived from the original on 7 December 2017. Retrieved 28 November 2017.
- ^ a b Smith, S. V.; Key, G. S. (1 May 1975). "Carbon dioxide and metabolism in marine environments1". Limnology and Oceanography. 20 (3): 493–495. Bibcode:1975LimOc..20..493S. doi:10.4319/lo.1975.20.3.0493. ISSN 1939-5590.
- ^ Rost, Björn; Riebesell, Ulf (2004). "Coccolithophores and the biological pump: Responses to environmental changes". Coccolithophores. Springer, Berlin, Heidelberg. pp. 99–125. CiteSeerX 10.1.1.455.2864. doi:10.1007/978-3-662-06278-4_5. ISBN 9783642060168.
- ^ Hain, M.P.; Sigman, D.M.; Haug, G.H (2014). "The Biological Pump in the Past". Treatise on Geochemistry. 8: 485–517. doi:10.1016/B978-0-08-095975-7.00618-5. ISBN 9780080983004.
- ^ a b Rost, Bjorn; Reibessel, Ulf (2004). Coccolithophores and the biological pump: responses to environmental changes. Berlin, Heidelberg: Springer. ISBN 978-3-642-06016-8.
- ^ Wilkinson, Owen & Carroll 1985
- ^ Wilkinson & Given 1986
- ^ Morse & Mackenzie 1990
- ^ a b c Hardie 1996
- ^ Lowenstein et al. 2001
- ^ Hardie 2003
- ^ Palmer & Wilson 2004
- ^ a b Ries, J. (2010). "Geological and experimental evidence for secular variation in seawater Mg/Ca (calcite-aragonite seas) and its effects on marine biological calcification". Biogeosciences. 7 (9): 2795–2849. Bibcode:2010BGeo....7.2795R. doi:10.5194/bg-7-2795-2010.
- ^ Adabi 2004
- ^ Ries, J. (2011). "Skeletal mineralogy in a high-CO2 world". Journal of Experimental Marine Biology and Ecology. 403 (1–2): 54–64. doi:10.1016/j.jembe.2011.04.006.
- ^ a b "Whiting Event, Lake Ontario". NASA Earth Observatory. 2 September 2013.
- ^ a b Larson, Erik B.; Mylroie, John E. (2014). "A review of whiting formation in the Bahamas and new models". Carbonates and Evaporites. 29 (4): 337–347. Bibcode:2014CarEv..29..337L. doi:10.1007/s13146-014-0212-7. ISSN 0891-2556. S2CID 128695792.
- ^ a b Sondi, Ivan; Juračić, Mladen (2010). "Whiting events and the formation of aragonite in Mediterranean Karstic Marine Lakes: new evidence on its biologically induced inorganic origin". Sedimentology. 57 (1): 85–95. Bibcode:2010Sedim..57...85S. doi:10.1111/j.1365-3091.2009.01090.x. ISSN 1365-3091. S2CID 129052529.
- ^ Long, Jacqueline S.; Hu, Chuanmin; Robbins, Lisa L.; Byrne, Robert H.; Paul, John H.; Wolny, Jennifer L. (2017). "Optical and biochemical properties of a southwest Florida whiting event". Estuarine, Coastal and Shelf Science. 196: 258–268. Bibcode:2017ECSS..196..258L. doi:10.1016/j.ecss.2017.07.017. ISSN 0272-7714.
- ^ Thompson, Joel B.; Schultze-Lam, Susanne; Beveridge, Terrance J.; Des Marais, David J. (1997). "Whiting events: Biogenic origin due to the photosynthetic activity of cyanobacterial picoplankton". Limnology and Oceanography. 42 (1): 133–41. Bibcode:1997LimOc..42..133S. doi:10.4319/lo.1997.42.1.0133. PMID 11541205. S2CID 139114.
- ^ "Whiting in Lake Michigan". NASA Earth Observatory. 18 September 2001.
- ^ Dittrich, Maria; Obst, Martin (2004). "Are Picoplankton Responsible for Calcite Precipitation in Lakes?". Ambio: A Journal of the Human Environment. 33 (8): 559–564. Bibcode:2004Ambio..33..559D. doi:10.1579/0044-7447-33.8.559. ISSN 0044-7447. PMID 15666689. S2CID 45359827.
- ^ Shinn, Eugene A.; St.C. Kendall, Christopher G. (1 December 2011). Day-Stirrat, Ruarri; Janson, Xavier; Wright, Wayne (eds.). "Back to the Future". The Sedimentary Record. 9 (4): 4–9. doi:10.2110/sedred.2011.4.4.
- ^ Yates, K.K; Robbins, L.L. (2001). "Microbial Lime-Mud Production and Its Relation to Climate Change". AAPG Studies in Geology. Tulsa, Ok: American Association of Petroleum Geologists. pp. 267–283.
- ^ Effler, Steven W.; Perkins, Mary Gail; Greer, Harry; Johnson, David L. (1987). "Effect of "whiting" on optical properties and turbidity in Owasco Lake, New York". Journal of the American Water Resources Association. 23 (2): 189–196. Bibcode:1987JAWRA..23..189E. doi:10.1111/j.1752-1688.1987.tb00796.x. ISSN 1093-474X.
- ^ a b c d e f g Smith, Helen E. K.; Poulton, Alex J.; Garley, Rebecca; Hopkins, Jason; Lubelczyk, Laura C.; Drapeau, Dave T.; Rauschenberg, Sara; Twining, Ben S.; Bates, Nicholas R.; Balch, William M. (2017). "The influence of environmental variability on the biogeography of coccolithophores and diatoms in the Great Calcite Belt". Biogeosciences. 14 (21): 4905–4925. Bibcode:2017BGeo...14.4905S. doi:10.5194/bg-14-4905-2017.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ a b c Balch, W. M.; Gordon, Howard R.; Bowler, B. C.; Drapeau, D. T.; Booth, E. S. (2005). "Calcium carbonate measurements in the surface global ocean based on Moderate-Resolution Imaging Spectroradiometer data". Journal of Geophysical Research. 110 (C7): C07001. Bibcode:2005JGRC..110.7001B. doi:10.1029/2004JC002560.
- ^ Sarmiento, Jorge L.; Hughes, Tertia M. C.; Stouffer, Ronald J.; Manabe, Syukuro (1998). "Simulated response of the ocean carbon cycle to anthropogenic climate warming". Nature. 393 (6682): 245–249. Bibcode:1998Natur.393..245S. doi:10.1038/30455. S2CID 4317429.
- ^ Sarmiento, J. L.; Slater, R.; Barber, R.; Bopp, L.; Doney, S. C.; Hirst, A. C.; Kleypas, J.; Matear, R.; Mikolajewicz, U.; Monfray, P.; Soldatov, V.; Spall, S. A.; Stouffer, R. (2004). "Response of ocean ecosystems to climate warming" (PDF). Global Biogeochemical Cycles. 18 (3): n/a. Bibcode:2004GBioC..18.3003S. doi:10.1029/2003GB002134. hdl:1912/3392. S2CID 15482539.
- ^ a b c Balch, W. M.; Drapeau, D. T.; Bowler, B. C.; Lyczskowski, E.; Booth, E. S.; Alley, D. (2011). "The contribution of coccolithophores to the optical and inorganic carbon budgets during the Southern Ocean Gas Exchange Experiment: New evidence in support of the "Great Calcite Belt" hypothesis". Journal of Geophysical Research. 116 (C4): C00F06. Bibcode:2011JGRC..116.0F06B. doi:10.1029/2011JC006941.
- ^ Sabine, C. L.; Feely, R. A.; Gruber, N.; Key, R. M.; Lee, K.; Bullister, J. L.; Wanninkhof, R.; Wong, C. S.; Wallace, D. W.; Tilbrook, B.; Millero, F. J.; Peng, T. H.; Kozyr, A.; Ono, T.; Rios, A. F. (2004). "The Oceanic Sink for Anthropogenic CO2" (PDF). Science. 305 (5682): 367–371. Bibcode:2004Sci...305..367S. doi:10.1126/science.1097403. hdl:10261/52596. PMID 15256665. S2CID 5607281.
- ^ a b Boyd, Philip W.; Strzepek, Robert; Fu, Feixue; Hutchins, David A. (2010). "Environmental control of open-ocean phytoplankton groups: Now and in the future". Limnology and Oceanography. 55 (3): 1353–1376. Bibcode:2010LimOc..55.1353B. doi:10.4319/lo.2010.55.3.1353.
- ^ Boyd, P. W.; Arrigo, K. R.; Strzepek, R.; Van Dijken, G. L. (2012). "Mapping phytoplankton iron utilization: Insights into Southern Ocean supply mechanisms". Journal of Geophysical Research: Oceans. 117 (C6): n/a. Bibcode:2012JGRC..117.6009B. doi:10.1029/2011JC007726.
- ^ a b Charalampopoulou, Anastasia; Poulton, Alex J.; Bakker, Dorothee C. E.; Lucas, Mike I.; Stinchcombe, Mark C.; Tyrrell, Toby (2016). "Environmental drivers of coccolithophore abundance and calcification across Drake Passage (Southern Ocean)". Biogeosciences. 13 (21): 5917–5935. Bibcode:2016BGeo...13.5917C. doi:10.5194/bg-13-5917-2016. hdl:11427/34237.
- ^ Boyd, P.W.; Newton, P.P. (1999). "Does planktonic community structure determine downward particulate organic carbon flux in different oceanic provinces?". Deep Sea Research Part I: Oceanographic Research Papers. 46 (1): 63–91. Bibcode:1999DSRI...46...63B. doi:10.1016/S0967-0637(98)00066-1.
- ^ a b Bathmann, U.V.; Scharek, R.; Klaas, C.; Dubischar, C.D.; Smetacek, V. (1997). "Spring development of phytoplankton biomass and composition in major water masses of the Atlantic sector of the Southern Ocean" (PDF). Deep Sea Research Part II: Topical Studies in Oceanography. 44 (1–2): 51–67. Bibcode:1997DSRII..44...51B. doi:10.1016/S0967-0645(96)00063-X.
- ^ a b c Poulton, Alex J.; Mark Moore, C.; Seeyave, Sophie; Lucas, Mike I.; Fielding, Sophie; Ward, Peter (2007). "Phytoplankton community composition around the Crozet Plateau, with emphasis on diatoms and Phaeocystis". Deep Sea Research Part II: Topical Studies in Oceanography. 54 (18–20): 2085–2105. Bibcode:2007DSRII..54.2085P. doi:10.1016/j.dsr2.2007.06.005.
- ^ a b Boyd, Philip W. (2002). "Environmental Factors Controlling Phytoplankton Processes in the Southern Ocean1". Journal of Phycology. 38 (5): 844–861. Bibcode:2002JPcgy..38..844B. doi:10.1046/j.1529-8817.2002.t01-1-01203.x. S2CID 53448178.
- ^ Balch, William M.; Bates, Nicholas R.; Lam, Phoebe J.; Twining, Benjamin S.; Rosengard, Sarah Z.; Bowler, Bruce C.; Drapeau, Dave T.; Garley, Rebecca; Lubelczyk, Laura C.; Mitchell, Catherine; Rauschenberg, Sara (2016). "Factors regulating the Great Calcite Belt in the Southern Ocean and its biogeochemical significance". Global Biogeochemical Cycles. 30 (8): 1124–1144. Bibcode:2016GBioC..30.1124B. doi:10.1002/2016GB005414. hdl:1912/8609. S2CID 22536090.
- ^ Barber, R. T.; Hiscock, M. R. (2006). "A rising tide lifts all phytoplankton: Growth response of other phytoplankton taxa in diatom-dominated blooms". Global Biogeochemical Cycles. 20 (4): n/a. Bibcode:2006GBioC..20.4S03B. doi:10.1029/2006GB002726.
- ^ Mohan, Rahul; Mergulhao, Lina P.; Guptha, M.V.S.; Rajakumar, A.; Thamban, M.; Anilkumar, N.; Sudhakar, M.; Ravindra, Rasik (2008). "Ecology of coccolithophores in the Indian sector of the Southern Ocean". Marine Micropaleontology. 67 (1–2): 30–45. Bibcode:2008MarMP..67...30M. doi:10.1016/j.marmicro.2007.08.005.
- ^ Holligan, P.M.; Charalampopoulou, A.; Hutson, R. (2010). "Seasonal distributions of the coccolithophore, Emiliania huxleyi, and of particulate inorganic carbon in surface waters of the Scotia Sea". Journal of Marine Systems. 82 (4): 195–205. Bibcode:2010JMS....82..195H. doi:10.1016/j.jmarsys.2010.05.007.
- ^ Cubillos, JC; Wright, SW; Nash, G.; De Salas, MF; Griffiths, B.; Tilbrook, B.; Poisson, A.; Hallegraeff, GM (2007). "Calcification morphotypes of the coccolithophorid Emiliania huxleyi in the Southern Ocean: Changes in 2001 to 2006 compared to historical data". Marine Ecology Progress Series. 348: 47–54. Bibcode:2007MEPS..348...47C. doi:10.3354/meps07058.
- ^ a b c Froneman, P.W.; McQuaid, C.D.; Perissinotto, R. (1995). "Biogeographic structure of the microphytoplankton assemblages of the south Atlantic and Southern Ocean during austral summer". Journal of Plankton Research. 17 (9): 1791–1802. doi:10.1093/plankt/17.9.1791.
- ^ a b c Hinz, D.J.; Poulton, A.J.; Nielsdóttir, M.C.; Steigenberger, S.; Korb, R.E.; Achterberg, E.P.; Bibby, T.S. (2012). "Comparative seasonal biogeography of mineralising nannoplankton in the Scotia Sea: Emiliania huxleyi, Fragilariopsis SPP. And Tetraparma pelagica". Deep Sea Research Part II: Topical Studies in Oceanography. 59–60: 57–66. Bibcode:2012DSRII..59...57H. doi:10.1016/j.dsr2.2011.09.002.
- ^ Langer, Gerald; Geisen, Markus; Baumann, Karl-Heinz; Kläs, Jessica; Riebesell, Ulf; Thoms, Silke; Young, Jeremy R. (2006). "Species-specific responses of calcifying algae to changing seawater carbonate chemistry" (PDF). Geochemistry, Geophysics, Geosystems. 7 (9): n/a. Bibcode:2006GGG.....7.9006L. doi:10.1029/2005GC001227. S2CID 14774230.
- ^ Tortell, Philippe D.; Payne, Christopher D.; Li, Yingyu; Trimborn, Scarlett; Rost, Björn; Smith, Walker O.; Riesselman, Christina; Dunbar, Robert B.; Sedwick, Pete; Ditullio, Giacomo R. (2008). "CO2sensitivity of Southern Ocean phytoplankton". Geophysical Research Letters. 35 (4): L04605. Bibcode:2008GeoRL..35.4605T. doi:10.1029/2007GL032583. S2CID 35741347.
- ^ Baines, Stephen B.; Twining, Benjamin S.; Brzezinski, Mark A.; Nelson, David M.; Fisher, Nicholas S. (2010). "Causes and biogeochemical implications of regional differences in silicification of marine diatoms". Global Biogeochemical Cycles. 24 (4): n/a. Bibcode:2010GBioC..24.4031B. doi:10.1029/2010GB003856.
- ^ Assmy, P.; Smetacek, V.; Montresor, M.; Klaas, C.; Henjes, J.; Strass, V. H.; Arrieta, J. M.; Bathmann, U.; Berg, G. M.; Breitbarth, E.; Cisewski, B.; Friedrichs, L.; Fuchs, N.; Herndl, G. J.; Jansen, S.; Kragefsky, S.; Latasa, M.; Peeken, I.; Rottgers, R.; Scharek, R.; Schuller, S. E.; Steigenberger, S.; Webb, A.; Wolf-Gladrow, D. (2013). "Thick-shelled, grazer-protected diatoms decouple ocean carbon and silicon cycles in the iron-limited Antarctic Circumpolar Current". Proceedings of the National Academy of Sciences. 110 (51): 20633–20638. Bibcode:2013PNAS..11020633A. doi:10.1073/pnas.1309345110. PMC 3870680. PMID 24248337.
- ^ Poulton, Alex J.; Painter, Stuart C.; Young, Jeremy R.; Bates, Nicholas R.; Bowler, Bruce; Drapeau, Dave; Lyczsckowski, Emily; Balch, William M. (2013). "The 2008Emiliania huxleyibloom along the Patagonian Shelf: Ecology, biogeochemistry, and cellular calcification". Global Biogeochemical Cycles. 27 (4): 1023–1033. Bibcode:2013GBioC..27.1023P. doi:10.1002/2013GB004641. S2CID 129706569.
- ^ Tsuchiya, Mizuki; Talley, Lynne D.; McCartney, Michael S. (1994). "Water-mass distributions in the western South Atlantic; A section from South Georgia Island (54S) northward across the equator". Journal of Marine Research. 52: 55–81. doi:10.1357/0022240943076759.
- ^ Orsi, Alejandro H.; Whitworth, Thomas; Nowlin, Worth D. (1995). "On the meridional extent and fronts of the Antarctic Circumpolar Current". Deep Sea Research Part I: Oceanographic Research Papers. 42 (5): 641–673. Bibcode:1995DSRI...42..641O. doi:10.1016/0967-0637(95)00021-W.
- ^ Belkin, Igor M.; Gordon, Arnold L. (1996). "Southern Ocean fronts from the Greenwich meridian to Tasmania". Journal of Geophysical Research: Oceans. 101 (C2): 3675–3696. Bibcode:1996JGR...101.3675B. doi:10.1029/95JC02750.
- ^ Signorini, Sergio R.; Garcia, Virginia M. T.; Piola, Alberto R.; Garcia, Carlos A. E.; Mata, Mauricio M.; McClain, Charles R. (2006). "Seasonal and interannual variability of calcite in the vicinity of the Patagonian shelf break (38°S–52°S)". Geophysical Research Letters. 33 (16): L16610. Bibcode:2006GeoRL..3316610S. doi:10.1029/2006GL026592.
- ^ Painter, Stuart C.; Poulton, Alex J.; Allen, John T.; Pidcock, Rosalind; Balch, William M. (2010). "The COPAS'08 expedition to the Patagonian Shelf: Physical and environmental conditions during the 2008 coccolithophore bloom". Continental Shelf Research. 30 (18): 1907–1923. Bibcode:2010CSR....30.1907P. doi:10.1016/j.csr.2010.08.013.
- ^ Sabine, C. L.; Feely, R. A.; Gruber, N.; Key, R. M.; Lee, K.; Bullister, J. L.; Wanninkhof, R.; Wong, C. S.; Wallace, D. W.; Tilbrook, B.; Millero, F. J.; Peng, T. H.; Kozyr, A.; Ono, T.; Rios, A. F. (2004). "The Oceanic Sink for Anthropogenic CO2" (PDF). Science. 305 (5682): 367–371. Bibcode:2004Sci...305..367S. doi:10.1126/science.1097403. hdl:10261/52596. PMID 15256665. S2CID 5607281.
- ^ a b Feely, R. A.; Sabine, C. L.; Lee, K.; Berelson, W.; Kleypas, J.; Fabry, V. J.; Millero, F. J. (2004). "Impact of Anthropogenic CO2 on the CaCO3 System in the Oceans". Science. 305 (5682): 362–366. Bibcode:2004Sci...305..362F. doi:10.1126/science.1097329. PMID 15256664. S2CID 31054160.
- ^ Meyer, J.; Riebesell, U. (2015). "Reviews and Syntheses: Responses of coccolithophores to ocean acidification: A meta-analysis". Biogeosciences. 12 (6): 1671–1682. Bibcode:2015BGeo...12.1671M. doi:10.5194/bg-12-1671-2015.
- ^ Riebesell, Ulf; Zondervan, Ingrid; Rost, Björn; Tortell, Philippe D.; Zeebe, Richard E.; Morel, François M. M. (2000). "Reduced calcification of marine plankton in response to increased atmospheric CO2" (PDF). Nature. 407 (6802): 364–367. Bibcode:2000Natur.407..364R. doi:10.1038/35030078. PMID 11014189. S2CID 4426501.
- ^ a b Armstrong, Robert A.; Lee, Cindy; Hedges, John I.; Honjo, Susumu; Wakeham, Stuart G. (2001). "A new, mechanistic model for organic carbon fluxes in the ocean based on the quantitative association of POC with ballast minerals". Deep Sea Research Part II: Topical Studies in Oceanography. 49 (1–3): 219–236. Bibcode:2001DSRII..49..219A. doi:10.1016/S0967-0645(01)00101-1.
- ^ Bach, Lennart T.; MacKinder, Luke C. M.; Schulz, Kai G.; Wheeler, Glen; Schroeder, Declan C.; Brownlee, Colin; Riebesell, Ulf (2013). "Dissecting the impact of CO 2 and pH on the mechanisms of photosynthesis and calcification in the coccolithophore Emiliania huxleyi" (PDF). New Phytologist. 199 (1): 121–134. doi:10.1111/nph.12225. PMID 23496417. S2CID 3661323.
- ^ a b Gafar, N. A.; Eyre, B. D.; Schulz, K. G. (2019). "Particulate inorganic to organic carbon production as a predictor for coccolithophorid sensitivity to ongoing ocean acidification". Limnology and Oceanography Letters. 4 (3): 62–70. Bibcode:2019LimOL...4...62G. doi:10.1002/lol2.10105.
- ^ Monteiro, Fanny M.; Bach, Lennart T.; Brownlee, Colin; Bown, Paul; Rickaby, Rosalind E. M.; Poulton, Alex J.; Tyrrell, Toby; Beaufort, Luc; Dutkiewicz, Stephanie; Gibbs, Samantha; Gutowska, Magdalena A.; Lee, Renee; Riebesell, Ulf; Young, Jeremy; Ridgwell, Andy (2016). "Why marine phytoplankton calcify". Science Advances. 2 (7): e1501822. Bibcode:2016SciA....2E1822M. doi:10.1126/sciadv.1501822. PMC 4956192. PMID 27453937.
- ^ a b Schlüter, Lothar; Lohbeck, Kai T.; Gutowska, Magdalena A.; Gröger, Joachim P.; Riebesell, Ulf; Reusch, Thorsten B. H. (2014). "Adaptation of a globally important coccolithophore to ocean warming and acidification". Nature Climate Change. 4 (11): 1024–1030. Bibcode:2014NatCC...4.1024S. doi:10.1038/nclimate2379.
- ^ Paasche, E. (2001). "A review of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae), with particular reference to growth, coccolith formation, and calcification-photosynthesis interactions". Phycologia. 40 (6): 503–529. Bibcode:2001Phyco..40..503P. doi:10.2216/i0031-8884-40-6-503.1. S2CID 84921998.
- ^ Lombard, Fabien; Guidi, Lionel; Kiørboe, Thomas (2013). "Effect of Type and Concentration of Ballasting Particles on Sinking Rate of Marine Snow Produced by the Appendicularian Oikopleura dioica". PLOS ONE. 8 (9): e75676. Bibcode:2013PLoSO...875676L. doi:10.1371/journal.pone.0075676. PMC 3783419. PMID 24086610.
- ^ Rost, Björn; Riebesell, Ulf (2004). "Coccolithophores and the biological pump: Responses to environmental changes". Coccolithophores. pp. 99–125. doi:10.1007/978-3-662-06278-4_5. ISBN 978-3-642-06016-8.
- ^ Beaufort, L.; Probert, I.; De Garidel-Thoron, T.; Bendif, E. M.; Ruiz-Pino, D.; Metzl, N.; Goyet, C.; Buchet, N.; Coupel, P.; Grelaud, M.; Rost, B.; Rickaby, R. E. M.; De Vargas, C. (2011). "Sensitivity of coccolithophores to carbonate chemistry and ocean acidification". Nature. 476 (7358): 80–83. doi:10.1038/nature10295. PMID 21814280. S2CID 4417285.
- ^ Hutchins, David A. (2011). "Forecasting the rain ratio". Nature. 476 (7358): 41–42. doi:10.1038/476041a. PMID 21814273.
- ^ Iglesias-Rodriguez, M. D.; Halloran, P. R.; Rickaby, R. E. M.; Hall, I. R.; Colmenero-Hidalgo, E.; Gittins, J. R.; Green, D. R. H.; Tyrrell, T.; Gibbs, S. J.; von Dassow, P.; Rehm, E.; Armbrust, E. V.; Boessenkool, K. P. (2008). "Phytoplankton Calcification in a High-CO2 World". Science. 320 (5874): 336–340. Bibcode:2008Sci...320..336I. doi:10.1126/science.1154122. PMID 18420926. S2CID 206511068.
- ^ De Bruijn, Douwe S.; Ter Braak, Paul M.; Van De Waal, Dedmer B.; Olthuis, Wouter; Van Den Berg, Albert (2021). "Coccolithophore calcification studied by single-cell impedance cytometry: Towards single-cell PIC:POC measurements". Biosensors and Bioelectronics. 173: 112808. doi:10.1016/j.bios.2020.112808. hdl:20.500.11755/aef0d454-d509-4620-89d0-a76e5a076bb6. PMID 33221507. S2CID 227135584.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Heldal, Mikal; Norland, Svein; Erichsen, Egil S.; Thingstad, T. Frede; Bratbak, Gunnar (2012). "An Unaccounted Fraction of Marine Biogenic CaCO3 Particles". PLOS ONE. 7 (10): e47887. Bibcode:2012PLoSO...747887H. doi:10.1371/journal.pone.0047887. PMC 3479124. PMID 23110119.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Sources
- Adabi, Mohammad H. (2004), "A re-evaluation of aragonite versus calcite seas", Carbonates and Evaporites, 19 (2): 133–141, Bibcode:2004CarEv..19..133A, doi:10.1007/BF03178476, S2CID 128955184
- Hardie, Lawrence A (1996), "Secular variation in seawater chemistry: An explanation for the coupled secular variation in the mineralogies of marine limestones and potash evaporites over the past 600 my", Geology, 24 (3), Geological Society of America: 279–283, Bibcode:1996Geo....24..279H, doi:10.1130/0091-7613(1996)024<0279:svisca>2.3.co;2
- Hardie, Lawrence A. (2003), "Secular variations in Precambrian seawater chemistry and the timing of Precambrian aragonite seas and calcite seas", Geology, 31 (9): 785–788, Bibcode:2003Geo....31..785H, doi:10.1130/g19657.1
- Lowenstein, T.K.; Timofeeff, M.N.; Brennan, S.T.; Hardie, L.A.; Demicco, R.V. (2001), "Oscillations in Phanerozoic seawater chemistry: evidence from fluid inclusions", Science, 294 (5544): 1086–1088, Bibcode:2001Sci...294.1086L, doi:10.1126/science.1064280, PMID 11691988, S2CID 2680231
- Morse, J.W.; Mackenzie, F.T. (1990). "Geochemistry of sedimentary carbonates". Developments in Sedimentology. 48: 1–707. doi:10.1016/S0070-4571(08)70330-3.
- Palmer, T.J.; Wilson, M.A. (2004). "Calcite precipitation and dissolution of biogenic aragonite in shallow Ordovician calcite seas". Lethaia. 37 (4): 417–427 [1]. Bibcode:2004Letha..37..417P. doi:10.1080/00241160410002135.
- Wilkinson, B.H.; Given, K.R. (1986). "Secular variation in abiotic marine carbonates: constraints on Phanerozoic atmospheric carbon dioxide contents and oceanic Mg/Ca ratios". Journal of Geology. 94 (3): 321–333. Bibcode:1986JG.....94..321W. doi:10.1086/629032. S2CID 128840375.
- Wilkinson, B.H.; Owen, R.M.; Carroll, A.R. (1985). "Submarine hydrothermal weathering, global eustacy, and carbonate polymorphism in Phanerozoic marine oolites". Journal of Sedimentary Petrology. 55: 171–183. doi:10.1306/212f8657-2b24-11d7-8648000102c1865d.


