Parasol cell
| Parasol cell | |
|---|---|
 Parasol cell general structure | |
| Details | |
| Part of | Retina of eye |
| System | Visual system |
| Identifiers | |
| FMA | 67916 |
| Anatomical terminology | |
A parasol cell, sometimes called an M cell[1] or M ganglion cell,[2] is one type of retinal ganglion cell (RGC) located in the ganglion cell layer of the retina. These cells project to magnocellular cells in the lateral geniculate nucleus (LGN) as part of the magnocellular pathway in the visual system.[3] They have large cell bodies as well as extensive branching dendrite networks and as such have large receptive fields.[4][3] Relative to other RGCs, they have fast conduction velocities.[4] While they do show clear center-surround antagonism (known as spatial opponency), they receive no information about color (absence of chromatic opponency).[3] Parasol ganglion cells contribute information about the motion and depth of objects to the visual system.[5]
Parasol ganglion cells in the Magnocellular pathway
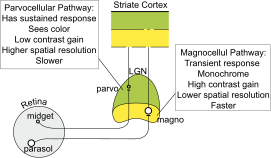
Parasol ganglion cells are the first step in the magnocellular pathway of the visual system. They project from the retina via the optic nerve to the two most ventral layers of the LGN, which is a nucleus of the thalamus, occupied by the magnocellular cells which then mainly project to the striate cortex (V1), typically to the layer 4Cα.[6]
Eventually, the information these cells collect in the retina is sent to various parts of the visual cortex, including the posterior parietal cortex and area V5 through the dorsal stream, and the inferior temporal cortex and area V4 through the ventral stream.[7]
Structure
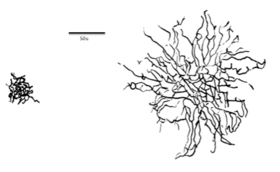
Parasol ganglion cells are located in the retina of the eyes, and make up roughly 10% of all retinal ganglion cells.[3] They have large bodies[4][6] with extensive, overlapping branched dendrites,[3][8] and thick, heavily myelinated axons. These properties allow parasol cells to conduct signals very quickly, much faster than the midget cells that feed the P pathway.[4][6]
Parasol ganglion cells collect information from large receptive fields,[3][6] containing both rods and cones.[9] Despite the input from cones, parasol ganglion cells do not receive information about color.[3][6] Unlike midget cells, parasol cell receptive fields contain the same color-type of cones in both their center and surround regions. Due to this lack of specificity, parasol cells cannot differentiate between different light wavelengths reflected from a specific object, and thus can only send achromatic information.[10]
There is approximately the same density of parasol ganglion cells in the fovea as in the rest of the retina, another property that distinguishes them from midget cells.[8]
Parasol vs. Midget cells
Parasol and midget retinal cells begin the parallel magnocellular and parvocellular pathways, respectively. While both parasol cells and midget cells play an important role in the visual system, their anatomies and functional contributions differ.[3][11][12][13]
| RGC Type | Parasol Cell | Midget Cell |
|---|---|---|
| Pathway it's involved in | Magnocellular Pathway | Parvocellular Pathway |
| Cell body size | Large | Small |
| Dendritic tree | Complex | Less complex |
| Conduction rate | ~1.6 ms | ~2 ms |
| Function in visual system | "Where" objects are; "How" to grasp the objects | "What" objects are according to fine detail |
| Sensitivity to spatial frequency | Low | Medium to high |
| Temporal frequency | High | Low |
| Color opponency | Achromatic | Red-green opponency |
Function
Parasol retinal ganglion cells cannot provide finely detailed or colored information,[4] but still provide useful static, depth, and motion information. Parasol ganglion cells have high light/dark contrast detection,[14] and are more sensitive at low spatial frequencies than high spatial frequencies. Due to this contrast information, these cells are good at detecting changes in luminance, and thus provide useful information for performing visual search tasks and detecting edges.[15]
Parasol retinal ganglion cells are also important for providing information about the location of objects. These cells can detect the orientation and position of objects in space,[5][12] information that will eventually be sent through the dorsal stream.[16] This information is also useful for detecting the difference in positions of objects on the retina of each eye, an important tool in binocular depth perception.[5][17]
Parasol cells have the ability to detect high temporal frequencies,[18] and can thus detect quick changes in the position of an object.[6] This is the basis for detecting motion.[5][14][19] The information sent to the intraparietal sulcus (IPS) of the posterior parietal cortex allows the magnocellular pathway to direct attention and guide saccadic eye movements to follow important moving objects in the visual field.[4][15][19] In addition to following objects with the eyes, the IPS sends information to parts of the frontal lobe that allows the hands and arms to adjust their movements to correctly grasp objects based on their size, position, and location.[16] This ability has led some neuroscientists to hypothesize that the purpose of the magnocellular pathway is not to detect spatial locations, but to guide actions related to the position and motion of objects.[20]
Research and experimentation
While neurons are typically studied by the extracellular use of metal electrodes, retinal ganglion cells are specifically studied in vitro. This method allows parasol cells' complicated and intertwined structure to be analyzed intracellularly. In 1941, Polyak was the first scientist to use Golgi staining to identify retinal ganglion cells. Here, dendritic morphology was closely analyzed and revealed large dendritic trees. Later in 1986, Kaplan and Shapley were then the first researchers to link parasol cells with the visual system. Recordings of S potentials at the axon terminals of RGCs in the LGN suggest that there is high contrast sensitivity in the cells terminating in the magnocellular layer of primates; opposed by low contrast sensitivity in cells found in the parvocellular layer.[3]

Primates and other model systems
Both old and new world primates have been used as model systems for human vision and have subsequently been beneficial in researching parasol cells.[8] Many retrograde labeling experiments using macaques, for example, have linked parasol and midget retinal ganglion cells with the magnocellular and parvocellular pathways respectively. In addition, similar studies have led to theories underlying color opponency.[3][8] Research by Dacey (1996) supports this idea where in vitro primate retinal cells were treated with dye fillings. Parasol cells of the magnocellular pathway were found to be achromatic.[3] In other studies, new world monkeys, such as marmosets, have aided in the current understanding of spatial and temporal frequency of the magnocellular layer in the LGN. Using the Nissl staining method, the magnocellular layer, in addition to the parvocellular layer, have darker and more dense cell bodies than the koniocellular layers, for example.[11]
Retinal ganglion cells of cats have been studied and compared to those in the visual system of both primates and humans. Evidence on receptive fields of cats confirms that parasol cell receptive fields are larger than those of midget cells because of their cellular structure. The same is likely to be found in human retinal cells which allows for better spatial localization.[3]
Associated disorders
Abnormal signalling in the magnocellular pathway has been associated with dyslexia and schizophrenia.[21][22]
Dyslexia
There is a theory that problems with underdeveloped parasol ganglion cells may contribute to causing dyslexia. Motion information contributed by parasol ganglion cells to the vision system helps the brain adjust the eyes in coordinated saccades, and problems in saccadic motion may lead to blurry vision and reading problems. This underdevelopment may be caused by several factors, including nutritional deficiencies and mutations in the KIAA0319 gene on chromosome six. Additionally, autoimmune attacks by antineuronal antibodies may prevent adequate parasol ganglion cell development for normal functioning, a theory which would explain why weakened immune systems are frequently present in dyslexic individuals.[4]
See also
References
- ^ Brodal, Per (2010). The central nervous system : structure and function (4th ed.). New York: Oxford University Press. pp. 226. ISBN 978-0-19-538115-3.
- ^ Gilbert, Scott F (2004). Purves, Dale (ed.). Neuroscience (3rd ed.). Sunderland, Mass.: Sinauer. p. 274. ISBN 978-0-87893-725-7.
- ^ a b c d e f g h i j k l Callaway EM (July 2005). "Structure and function of parallel pathways in the primate early visual system". The Journal of Physiology. 566 (Pt 1): 13–9. doi:10.1113/jphysiol.2005.088047. PMC 1464718. PMID 15905213.
- ^ a b c d e f g Stein J (2014-01-01). "Dyslexia: the Role of Vision and Visual Attention". Current Developmental Disorders Reports. 1 (4): 267–280. doi:10.1007/s40474-014-0030-6. PMC 4203994. PMID 25346883.
- ^ a b c d Atkinson, J. (1992-01-01). "Early visual development: differential functioning of parvocellular and magnocellular pathways". Eye. 6 ( Pt 2) (2): 129–135. doi:10.1038/eye.1992.28. PMID 1624034.
- ^ a b c d e f Nassi JJ, Callaway EM (May 2009). "Parallel processing strategies of the primate visual system". Nature Reviews. Neuroscience. 10 (5): 360–72. doi:10.1038/nrn2619. PMC 2771435. PMID 19352403.
- ^ Yabuta, N. H.; Sawatari, A.; Callaway, E. M. (2001-04-13). "Two functional channels from primary visual cortex to dorsal visual cortical areas". Science. 292 (5515): 297–300. Bibcode:2001Sci...292..297Y. CiteSeerX 10.1.1.224.6907. doi:10.1126/science.1057916. ISSN 0036-8075. PMID 11303106. S2CID 1360928.
- ^ a b c d Lee BB (March 1996). "Receptive field structure in the primate retina". Vision Research. 36 (5): 631–44. doi:10.1016/0042-6989(95)00167-0. PMID 8762295.
- ^ Hadjikhani N, Tootell RB (2000-01-01). "Projection of rods and cones within human visual cortex". Human Brain Mapping. 9 (1): 55–63. doi:10.1002/(sici)1097-0193(2000)9:1<55::aid-hbm6>3.0.co;2-u. PMC 6871842. PMID 10643730.
- ^ Sincich, Lawrence C.; Horton, Jonathan C. (2005-01-01). "The circuitry of V1 and V2: integration of color, form, and motion". Annual Review of Neuroscience. 28: 303–326. doi:10.1146/annurev.neuro.28.061604.135731. ISSN 0147-006X. PMID 16022598.
- ^ a b Jayakumar J, Dreher B, Vidyasagar TR (May 2013). "Tracking blue cone signals in the primate brain". Clinical & Experimental Optometry. 96 (3): 259–66. doi:10.1111/j.1444-0938.2012.00819.x. hdl:11343/39658. PMID 23186138. S2CID 32259651.
- ^ a b Skottun, Bernt C.; Skoyles, John R. (2011-01-01). "On identifying magnocellular and parvocellular responses on the basis of contrast-response functions". Schizophrenia Bulletin. 37 (1): 23–26. doi:10.1093/schbul/sbq114. PMC 3004196. PMID 20929967.
- ^ Skoyles J, Skottun BC (January 2004). "On the prevalence of magnocellular deficits in the visual system of non-dyslexic individuals". Brain and Language. 88 (1): 79–82. doi:10.1016/s0093-934x(03)00162-7. PMID 14698733. S2CID 19152620.
- ^ a b Pokorny, Joel (2011-07-07). "Review: steady and pulsed pedestals, the how and why of post-receptoral pathway separation". Journal of Vision. 11 (5): 7. doi:10.1167/11.5.7. PMID 21737512.
- ^ a b Cheng, Alicia; Eysel, Ulf T.; Vidyasagar, Trichur R. (2004-10-01). "The role of the magnocellular pathway in serial deployment of visual attention". The European Journal of Neuroscience. 20 (8): 2188–2192. doi:10.1111/j.1460-9568.2004.03675.x. PMID 15450098. S2CID 31456201.
- ^ a b Hebart, Martin N.; Hesselmann, Guido (2012-06-13). "What visual information is processed in the human dorsal stream?". The Journal of Neuroscience. 32 (24): 8107–8109. doi:10.1523/JNEUROSCI.1462-12.2012. PMC 6703654. PMID 22699890.
- ^ Poggio, G.F.; Poggio, T. (1984). "The analysis of stereopsis". Annual Review of Neuroscience. 7: 379–412. doi:10.1146/annurev.ne.07.030184.002115. PMID 6370081.
- ^ Anderson, Andrew J.; Jiao, Julie; Bui, Bang V. (2015-09-01). "Efficiently Measuring Magnocellular and Parvocellular Function in Human Clinical Studies". Translational Vision Science & Technology. 4 (5): 1. doi:10.1167/tvst.4.5.1. PMC 4559216. PMID 26346944.
- ^ a b Vidyasagar, Trichur R. (2004-01-01). "Neural underpinnings of dyslexia as a disorder of visuo-spatial attention". Clinical & Experimental Optometry. 87 (1): 4–10. doi:10.1111/j.1444-0938.2004.tb03138.x. PMID 14720113. S2CID 45931933.
- ^ Goodale, M.A.; Westwood, D.A. (2004). "An evolving view of duplex vision: separate but interacting cortical pathways for perception and action". Current Opinion in Neurobiology. 14 (2): 203–211. doi:10.1016/j.conb.2004.03.002. PMID 15082326. S2CID 15917985.
- ^ Stein, John (2014-01-01). "Dyslexia: the Role of Vision and Visual Attention". Current Developmental Disorders Reports. 1 (4): 267–280. doi:10.1007/s40474-014-0030-6. ISSN 2196-2987. PMC 4203994. PMID 25346883.
- ^ Bortolon, Catherine; Capdevielle, Delphine; Raffard, Stéphane (2015-06-01). "Face recognition in schizophrenia disorder: A comprehensive review of behavioral, neuroimaging and neurophysiological studies". Neuroscience and Biobehavioral Reviews. 53: 79–107. doi:10.1016/j.neubiorev.2015.03.006. ISSN 1873-7528. PMID 25800172. S2CID 20887067.
