Oxetacaine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, topical |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 1 hour |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.346 |
| Chemical and physical data | |
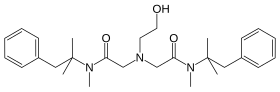
| Formula | C28H41N3O3 |
| Molar mass | 467.654 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Oxetacaine (INN, also known as oxethazaine) is a potent local anesthetic. It is administered orally (usually in combination with an antacid) for the relief of pain associated with peptic ulcer disease or esophagitis. It is also used topically in the management of hemorrhoid pain. Oral oxetacaine preparations are available in several countries, including India, South Africa, Japan, Taiwan and Brazil, but not the United States. Unlike most local anesthetics, oxetacaine does not break down under strongly acidic conditions.[1]
It is known to produce mephentermine and phentermine as metabolites.[2][3]
References
- ^ Seifter J, Glassman JM, Hudyma GM (1962). "Oxethazaine and related congeners: a series of highly potent local anesthetics". Proc Soc Exp Biol Med. 109 (3): 664–8. doi:10.3181/00379727-109-27300. PMID 13910333. S2CID 39641018.
- ^ Hsu MC, Lin SF, Kuan CP, Chu WL, Chan KH, Chang-Chien GP (March 2009). "Oxethazaine as the source of mephentermine and phentermine in athlete's urine". Forensic Sci Int. 185 (1–3): e1–5. doi:10.1016/j.forsciint.2008.12.009. PMID 19157735.
- ^ Huang WH, Liu CH, Liu RH, Tseng YL (March 2010). "Confirming urinary excretion of mephentermine and phentermine following the ingestion of oxethazaine by gas chromatography-mass spectrometry analysis". J Anal Toxicol. 34 (2): 73–77. doi:10.1093/jat/34.2.73. PMID 20223098.
External links
