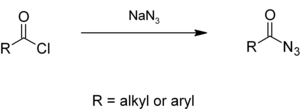Organic azide

An organic azide is an organic compound that contains an azide (–N3) functional group.[1] Because of the hazards associated with their use, few azides are used commercially although they exhibit interesting reactivity for researchers. Low molecular weight azides are considered especially hazardous and are avoided. In the research laboratory, azides are precursors to amines. They are also popular for their participation in the "click reaction" between an azide and an alkyne and in Staudinger ligation. These two reactions are generally quite reliable, lending themselves to combinatorial chemistry.
History
Phenyl azide ("diazoamidobenzol"), was prepared in 1864 by Peter Griess by the reaction of ammonia and phenyldiazonium.[2][3] In the 1890s, Theodor Curtius, who had discovered hydrazoic acid (HN3), described the rearrangement of acyl azides to isocyanates subsequently named the Curtius rearrangement.[4] Rolf Huisgen described the eponymous 1,3-dipolar cycloaddition.[5][6]
The interest in azides among organic chemists has been relatively modest due to the reported instability of these compounds.[7] The situation has changed dramatically with the discovery by Sharpless et al. of Cu-catalysed (3+2)-cycloadditions between organic azides and terminal alkynes.[8][9] The azido- and the alkyne groups are "bioorthogonal", which means they do not interact with living systems, and at the same time they undergo an impressively fast and selective coupling. This type of formal 1,3-dipolar cycloaddition became the most famous example of so-called "click chemistry"[10][11] (perhaps, the only one known to a non-specialist), and the field of organic azides exploded.
Preparation
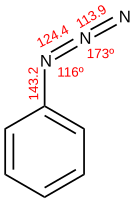
Myriad methods exist, most often using preformed azide-containing reagent.

Alkyl azides
By halide displacement
As a pseudohalide, azide generally displaces many leaving group, e.g. Br−, I−, TsO−, sulfonate,[13][14] and others to give the azido compound.[15] The azide source is most often sodium azide (NaN3), although lithium azide (LiN3) has been demonstrated.
From alcohols
Aliphatic alcohols give azides via a variant of the Mitsunobu reaction, with the use of hydrazoic acid.[1] Hydrazines may also form azides by reaction with sodium nitrite:[16] Alcohols can be converted into azides in one step using 2-azido-1,3-dimethylimidazolinium hexafluorophosphate (ADMP)[17] or under Mitsunobu conditions[18] with diphenylphosphoryl azide (DPPA).
From epoxides and aziridines
Trimethylsilyl azide (CH3)3SiN3, and tributyltin azide (CH3CH2CH2CH2)3SnN3, have all been used,[7] including enantioselective[19] modifications of the reaction are also known. Aminoazides are accessible by the epoxide and aziridine ring cleavage, respectively.[20][21]
From amines
The azo-transfer compounds, trifluoromethanesulfonyl azide and imidazole-1-sulfonyl azide react with amines to give the corresponding azides. Diazo transfer onto amines using trifluoromethanesulfonyl azide (TfN3) and Tosyl azide (TsN3) has been reported.[22]
Hydroazidation
Hydroazidation of alkenes has been demonstrated[23]
Aryl azides
Aryl azides may be prepared by displacement of the appropriate diazonium salt with sodium azide or trimethylsilyl azide. Nucleophilic aromatic substitution is also possible, even with chlorides. Anilines and aromatic hydrazines undergo diazotization, as do alkyl amines and hydrazines.[1]
Acyl azides
Alkyl or aryl acyl chlorides react with sodium azide in aqueous solution to give acyl azides,[24][25] which give isocyanates in the Curtius rearrangement.
Dutt–Wormall reaction
A classic method for the synthesis of azides is the Dutt–Wormall reaction[26] in which a diazonium salt reacts with a sulfonamide first to a diazoaminosulfinate and then on hydrolysis the azide and a sulfinic acid.[27]
Reactions
Organic azides engage in useful organic reactions. The terminal nitrogen is mildly nucleophilic. Generally, nucleophiles attack the azide at the terminal nitrogen Nγ, while electrophiles react at the internal atom Nα.[28] Azides easily extrude diatomic nitrogen, a tendency that is exploited in many reactions such as the Staudinger ligation or the Curtius rearrangement.[29]
Azides may be reduced to amines by hydrogenolysis[30] or with a phosphine (e.g., triphenylphosphine) in the Staudinger reaction. This reaction allows azides to serve as protected -NH2 synthons, as illustrated by the synthesis of 1,1,1-tris(aminomethyl)ethane:
- 3 H2 + CH3C(CH2N3)3 → CH3C(CH2NH2)3 + 3 N2
In the azide alkyne Huisgen cycloaddition, organic azides react as 1,3-dipoles, reacting with alkynes to give substituted 1,2,3-triazoles.
Some azide reactions are shown in the following scheme. Probably the most famous is the reaction with phosphines, which leads to iminophosphoranes 22; these can be hydrolysed into primary amines 23 (the Staudinger reaction),[31] react with carbonyl compounds to give imines 24 (the aza-Wittig reaction),[32][33][34] or undergo other transformations. Thermal decomposition of azides gives nitrenes, which participate in a variety of reactions; vinyl azides 19 decompose into 2H-azirines 20.[28][35] Alkyl azides with low nitrogen-content ((nC + nO) / nN ≥ 3) are relatively stable and decompose only above ca. 175 °C.[36]
Direct photochemical decomposition of alkyl azides leads almost exclusively to imines (e.g. 25 and 26).[28] It is proposed that the azide group is promoted to the singlet excited state and then undergoes concerted rearrangement without the intermediacy of nitrenes. The presence of triplet sensitisers, however, may change the reaction mechanism and result in the formation of triplet nitrenes. The latter were observed directly by ESR spectroscopy at −269 °C as well as inferred in some photolyses.[37][38] Triplet methyl nitrene is 31 kJ/mol more stable than its singlet form, and thus is most likely the ground state.[28][39]
The (3+2)-cycloaddition of azides to double or triple bonds is one of the most utilised cycloadditions in organic chemistry and affords triazolines (e.g. 17) or triazoles, respectively.[40][41][42] The uncatalysed reaction is a concerted pericyclic process, in which the configuration of the alkene component is transferred to the triazoline product. The Woodward–Hoffmann denomination is [π4s+π2s] and the reaction is symmetry-allowed. According to Sustmann, this is a Type II cycloaddition, which means the two HOMOs and the two LUMOs have comparable energies, and thus both electron-withdrawing and electron-donating substituents may lead to an increase in the reaction rate.[43][44] The reaction is generally free from significant solvent effects because both the reactants and the transition state (TS) are non-polar.[45]
Another azide regular is tosyl azide here in reaction with norbornadiene in a nitrogen insertion reaction:[46]
Applications
Some azides are valuable as bioorthogonal chemical reporters, molecules that can be "clicked" to see the metabolic path it has taken inside a living system.
The antiviral drug zidovudine (AZT) contains an azido group.
Safety
Some organic azides are classified as highly explosive and toxic.[47]
References
- ^ a b c S. Bräse; C. Gil; K. Knepper; V. Zimmermann (2005). "Organic Azides: An Exploding Diversity of a Unique Class of Compounds". Angewandte Chemie International Edition. 44 (33): 5188–5240. doi:10.1002/anie.200400657. PMID 16100733.
- ^ Griess, John Peter; Hofmann, August Wilhelm Von (1864-01-01). "XX. On a new class of compounds in which nitrogen is substituted for hydrogen". Proceedings of the Royal Society of London. 13: 375–384. doi:10.1098/rspl.1863.0082. S2CID 94746575.
- ^ Griess, Peter (1866). "Ueber eine neue Klasse organischer Verbindungen, in denen Wasserstoff durch Stickstoff vertreten ist". Annalen der Chemie und Pharmacie (in German). 137 (1): 39–91. doi:10.1002/jlac.18661370105.
- ^ Jay, R.; Curtius, Th. (January 1894). "Zur Reduction des Diazoessigesters". Berichte der Deutschen Chemischen Gesellschaft (in German). 27 (1): 775–778. doi:10.1002/cber.189402701151.
- ^ Huisgen, Rolf (October 1963). "1,3-Dipolar Cycloadditions. Past and Future". Angewandte Chemie International Edition in English. 2 (10): 565–598. doi:10.1002/anie.196305651. ISSN 0570-0833.
- ^ Huisgen, R. (November 1963). "Kinetics and Mechanism of 1,3-Dipolr Cycloadditions". Angewandte Chemie International Edition in English. 2 (11): 633–645. doi:10.1002/anie.196306331. ISSN 0570-0833.
- ^ a b Organic azides: syntheses and applications. Stefan Bräse, Klaus Banert. Chichester, West Sussex, U.K.: John Wiley. 2010. ISBN 978-0-470-68252-4. OCLC 587390490.
{{cite book}}: CS1 maint: others (link) - ^ Demko, Zachary P.; Sharpless, K. Barry (November 2001). "Preparation of 5-Substituted 1 H -Tetrazoles from Nitriles in Water †". The Journal of Organic Chemistry. 66 (24): 7945–7950. doi:10.1021/jo010635w. ISSN 0022-3263. PMID 11722189.
- ^ Kolb, Hartmuth C.; Finn, M. G.; Sharpless, K. Barry (2001). "Click Chemistry: Diverse Chemical Function from a Few Good Reactions". Angewandte Chemie International Edition. 40 (11): 2004–2021. doi:10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. ISSN 1521-3773. PMID 11433435.
- ^ Binder, Wolfgang; Kluger, Christian (2006-09-01). "Azide/Alkyne-"Click" Reactions: Applications in Material Science and Organic Synthesis". Current Organic Chemistry. 10 (14): 1791–1815. doi:10.2174/138527206778249838.
- ^ Rostovtsev, Vsevolod V.; Green, Luke G.; Fokin, Valery V.; Sharpless, K. Barry (2002). "A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective "Ligation" of Azides and Terminal Alkynes". Angewandte Chemie International Edition. 41 (14): 2596–2599. doi:10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. ISSN 1521-3773. PMID 12203546.
- ^ Wagner, Gerald; Arion, Vladimir B.; Brecker, Lothar; Krantz, Carsten; Mieusset, Jean-Luc; Brinker, Udo H. (2009). "Controllable Selective Functionalization of a Cavitand via Solid State Photolysis of an Encapsulated Phenyl Azide". Organic Letters. 11 (14): 3056–3058. doi:10.1021/ol901122h. PMID 19537769.
- ^ Baran, Phil S.; Zografos, Alexandros L.; O'Malley, Daniel P. (March 2004). "Short Total Synthesis of (±)-Sceptrin". Journal of the American Chemical Society. 126 (12): 3726–3727. doi:10.1021/ja049648s. ISSN 0002-7863. PMID 15038721.
- ^ Shaffer, Karl J.; Taylor, Carol M. (2006-08-01). "β-Glycosides of Hydroxyproline via an Umpolung Approach". Organic Letters. 8 (18): 3959–3962. doi:10.1021/ol061424m. ISSN 1523-7060. PMID 16928048.
- ^ Righi, Giuliana; D'Achille, Claudia; Pescatore, Giovanna; Bonini, Carlo (September 2003). "New stereoselective synthesis of the peptidic aminopeptidase inhibitors bestatin, phebestin and probestin". Tetrahedron Letters. 44 (37): 6999–7002. doi:10.1016/S0040-4039(03)01799-4.
- ^ R. O. Lindsay and C. F. H. Allen (1942). "Phenyl azide". Organic Syntheses. 22: 96. doi:10.15227/orgsyn.022.0096.
- ^ Kitamura, Mitsuru; Koga, Tatsuya; Yano, Masakazu; Okauchi, Tatsuo (June 2012). "Direct Synthesis of Organic Azides from Alcohols Using 2-Azido-1,3-dimethylimidazolinium Hexafluorophosphate". Synlett. 23 (9): 1335–1338. doi:10.1055/s-0031-1290958. ISSN 0936-5214.
- ^ Lee, Sang-Hyeup; Yoon, Juyoung; Chung, Seung-Hwan; Lee, Yoon-Sik (March 2001). "Efficient asymmetric synthesis of 2,3-diamino-3-phenylpropanoic acid derivatives". Tetrahedron. 57 (11): 2139–2145. doi:10.1016/S0040-4020(01)00090-4.
- ^ Martinez, Luis E.; Leighton, James L.; Carsten, Douglas H.; Jacobsen, Eric N. (May 1995). "Highly Enantioselective Ring Opening of Epoxides Catalyzed by (salen)Cr(III) Complexes". Journal of the American Chemical Society. 117 (21): 5897–5898. doi:10.1021/ja00126a048. ISSN 0002-7863.
- ^ Sabitha, Gowravaram; Babu, R. Satheesh; Rajkumar, M.; Yadav, J. S. (February 2002). "Cerium(III) Chloride Promoted Highly Regioselective Ring Opening of Epoxides and Aziridines Using NaN 3 in Acetonitrile: A Facile Synthesis of 1,2-Azidoalcohols and 1,2-Azidoamines †". Organic Letters. 4 (3): 343–345. doi:10.1021/ol016979q. ISSN 1523-7060. PMID 11820875.
- ^ Saito, Seiki; Bunya, Norio; Inaba, Masami; Moriwake, Toshio; Torii, Sigeru (January 1985). "A facile cleavage of oxirane with hydrazoic acid in dmf A new route to chiral β-hydroxy-α-amino acids". Tetrahedron Letters. 26 (43): 5309–5312. doi:10.1016/S0040-4039(00)95024-X.
- ^ Titz, Alexander; Radic, Zorana; Schwardt, Oliver; Ernst, Beat (April 2006). "A safe and convenient method for the preparation of triflyl azide, and its use in diazo transfer reactions to primary amines". Tetrahedron Letters. 47 (14): 2383–2385. doi:10.1016/j.tetlet.2006.01.157.
- ^ Waser, Jérôme; Gaspar, Boris; Nambu, Hisanori; Carreira, Erick M. (2006-09-01). "Hydrazines and Azides via the Metal-Catalyzed Hydrohydrazination and Hydroazidation of Olefins". Journal of the American Chemical Society. 128 (35): 11693–11712. doi:10.1021/ja062355+. ISSN 0002-7863. PMID 16939295.
- ^ C. F. H. Allen; Alan Bell. "Undecyl isocyanate". Organic Syntheses; Collected Volumes, vol. 3, p. 846.
- ^ Jon Munch-Petersen (1963). "m-Nitrobenzazide". Organic Syntheses; Collected Volumes, vol. 4, p. 715.
- ^ Pavitra Kumar Dutt; Hugh Robinson Whitehead & Arthur Wormall (1921). "CCXLI.—The action of diazo-salts on aromatic sulphonamides. Part I". J. Chem. Soc., Trans. 119: 2088–2094. doi:10.1039/CT9211902088.
- ^ Name Reactions: A Collection of Detailed Reaction Mechanisms by Jie Jack Li Published 2003 Springer ISBN 3-540-40203-9
- ^ a b c d Organic azides : syntheses and applications. Stefan Bräse, Klaus Banert. Chichester, West Sussex, U.K.: John Wiley. 2010. p. 507. ISBN 978-0-470-68252-4. OCLC 587390490.
{{cite book}}: CS1 maint: others (link) - ^ Saul Patai, ed. (1971). The Azido Group. PATAI'S Chemistry of Functional Groups. doi:10.1002/9780470771266. ISBN 9780470771266.
- ^ "Amine synthesis by azide reduction".
- ^ Gololobov, Yuri G.; Kasukhin, Leonid F. (February 1992). "Recent advances in the staudinger reaction". Tetrahedron. 48 (8): 1353–1406. doi:10.1016/S0040-4020(01)92229-X.
- ^ Molina, Pedro; Vilaplana, Maria Jesús (1994). "Iminophosphoranes: Useful Building Blocks for the Preparation of Nitrogen-Containing Heterocycles". Synthesis. 1994 (12): 1197–1218. doi:10.1055/s-1994-25672. ISSN 0039-7881. S2CID 196726458.
- ^ Fresneda, Pilar M.; Molina, Pedro (2004). "Application of Iminophosphorane-Based Methodologies for the Synthesis of Natural Products". Synlett. 2004 (1): 1–17. doi:10.1055/s-2003-43338. ISSN 0936-5214.
- ^ Palacios, Francisco; Alonso, Concepción; Aparicio, Domitila; Rubiales, Gloria; de los Santos, Jesús M. (January 2007). "The aza-Wittig reaction: an efficient tool for the construction of carbon–nitrogen double bonds". Tetrahedron. 63 (3): 523–575. doi:10.1016/j.tet.2006.09.048.
- ^ Álvares, Yolanda S. P.; Alves, M. José; Azoia, Nuno G.; Bickley, Jamie F.; Gilchrist, Thomas L. (2002). "Diastereoselective synthesis of aziridines from (1R)-10-(N,N-dialkylsulfamoyl)isobornyl 2H-azirine-3-carboxylates". J. Chem. Soc., Perkin Trans. 1 (16): 1911–1919. doi:10.1039/B202321K. ISSN 1472-7781.
- ^ The chemistry of the azido group. Saul Patai. London: Interscience Publishers. 1971. p. 626. ISBN 978-0-470-77126-6. OCLC 501315944.
{{cite book}}: CS1 maint: others (link) - ^ Wasserman, E.; Smolinsky, G.; Yager, W. A. (August 1964). "Electron Spin Resonance of Alkyl Nitrenes". Journal of the American Chemical Society. 86 (15): 3166–3167. doi:10.1021/ja01069a049. ISSN 0002-7863.
- ^ Klima, Rodney F; Gudmundsdóttir, Anna D (March 2004). "Intermolecular triplet-sensitized photolysis of alkyl azides". Journal of Photochemistry and Photobiology A: Chemistry. 162 (2–3): 239–247. doi:10.1016/S1010-6030(03)00368-X.
- ^ Travers, Michael J.; Cowles, Daniel C.; Clifford, Eileen P.; Ellison, G. Barney; Engelking, Paul C. (1999-09-22). "Photoelectron spectroscopy of the CH3N− ion". The Journal of Chemical Physics. 111 (12): 5349–5360. Bibcode:1999JChPh.111.5349T. doi:10.1063/1.479795. ISSN 0021-9606.
- ^ Synthetic applications of 1,3-dipolar cycloaddition chemistry toward heterocycles and natural products. Albert Padwa, William H. Pearson. Hoboken, NJ: Wiley. 2003. p. 940. ISBN 0-471-28061-5. OCLC 51312904.
{{cite book}}: CS1 maint: others (link) - ^ Bräse, Stefan (October 2004). "The Virtue of the Multifunctional Triazene Linkers in the Efficient Solid-Phase Synthesis of Heterocycle Libraries". Accounts of Chemical Research. 37 (10): 805–816. doi:10.1021/ar0200145. ISSN 0001-4842. PMID 15491127.
- ^ Tarabara, I. N.; Kas'yan, A. O.; Yarovoi, M. Yu.; Shishkina, S. V.; Shishkin, O. V.; Kas'yan, L. I. (July 2004). "Reactions of Bicyclo[2.2.1]hept-5-ene-2,3-dicarboximides with Aromatic Azides". Russian Journal of Organic Chemistry. 40 (7): 992–998. doi:10.1023/B:RUJO.0000045191.12939.47. ISSN 1070-4280. S2CID 97421265.
- ^ Sustmann, Reiner (January 1971). "A simple model for substituent effects in cycloaddition reactions. I. 1,3-dipolar cycloadditions". Tetrahedron Letters. 12 (29): 2717–2720. doi:10.1016/S0040-4039(01)96961-8.
- ^ Sustmann, R. (1974-01-01). "Orbital energy control of cycloaddition reactivity". Pure and Applied Chemistry. 40 (4): 569–593. doi:10.1351/pac197440040569. ISSN 1365-3075. S2CID 28715256.
- ^ Huisgen, Rolf; Geittner, Jochen; Reissig, Hans-Ulrich (1978). "Solvent Dependence of Cycloaddition Rates of Phenyldiazomethane and Activation Parameters". Heterocycles. 11 (1): 109. doi:10.3987/S(N)-1978-01-0109. ISSN 0385-5414.
- ^ Damon D. Reed & Stephen C. Bergmeier (2007). "A Facile Synthesis of a Polyhydroxylated 2-Azabicyclo[3.2.1]octane". J. Org. Chem. 72 (3): 1024–6. doi:10.1021/jo0619231. PMID 17253828.
- ^ Treitler, Daniel S.; Leung, Simon (2 September 2022). "How Dangerous Is Too Dangerous? A Perspective on Azide Chemistry". The Journal of Organic Chemistry. 87 (17): 11293–11295. doi:10.1021/acs.joc.2c01402. ISSN 0022-3263. PMID 36052475. S2CID 252009657. Retrieved 18 September 2022.
![]() This article incorporates text by Oleksandr Zhurakovskyi available under the CC BY 2.5 license.
This article incorporates text by Oleksandr Zhurakovskyi available under the CC BY 2.5 license.
Additional sources
- Patai, Saul, ed. (1971-01-01). The Azido Group (1971). PATai's Chemistry of Functional Groups. Chichester, UK: John Wiley & Sons, Ltd. p. 626. doi:10.1002/9780470771266. ISBN 978-0-470-77126-6.
- Scriven, Eric F. Azides and Nitrenes: Reactivity and Utility. Academic Press. p. 542. ISBN 9780124143074.
- Padwa, Albert; Pearson, William H., eds. (2002-04-05). Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products: Padwa/Dipolar Cycloaddition E-Bk. Chemistry of Heterocyclic Compounds: A Series Of Monographs. Vol. 59. New York, USA: John Wiley & Sons, Inc. doi:10.1002/0471221902. ISBN 978-0-471-38726-8.
- Wolff, H. Org. React. 1946, 3, 337–349.
- Boyer, J. H.; Canter, F. C. (1954-02-01). "Alkyl and Aryl Azides". Chemical Reviews. 54 (1): 1–57. doi:10.1021/cr60167a001. ISSN 0009-2665.
- Scriven, Eric F. V.; Turnbull, Kenneth (March 1988). "Azides: Their Preparation and synthetic uses". Chemical Reviews. 88 (2): 297–368. doi:10.1021/cr00084a001. ISSN 0009-2665.
- Bräse, Stefan; Gil, Carmen; Knepper, Kerstin; Zimmermann, Viktor (2005-08-19). "Organic Azides: An Exploding Diversity of a Unique Class of Compounds". Angewandte Chemie International Edition. 44 (33): 5188–5240. doi:10.1002/anie.200400657. ISSN 1433-7851. PMID 16100733.