Onfasprodil
 | |
| Clinical data | |
|---|---|
| Other names | MIJ821 |
| Drug class | NMDA receptor modulator |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
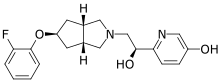
| Formula | C20H23FN2O3 |
| Molar mass | 358.413 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Onfasprodil (MIJ821) is a drug delivered via intravenous infusion that is designed as a fast-acting treatment for treatment-resistant depression. It works as a negative allosteric modulator of the NMDA receptor subunit 2B (NR2B). The drug is developed by Novartis.[1][2]
References
- ^ Gomez-Mancilla, Baltazar; Levy, Jeffrey A.; Ganesan, Subramanian; Faller, Thomas; Issachar, Gil; Peremen, Ziv; Laufer, Offir; Shani-Hershkovich, Revital; Biliouris, Kostas; Walker, Ela; Healy, Mark P.; Sverdlov, Oleksandr; Desai, Sachin; Ghaemi, S. Nassir; Cha, Jang-Ho; Shanker, Y. Gopi (November 2023). "MIJ821 (onfasprodil) in healthy volunteers: First-in-human, randomized, placebo-controlled study (single ascending dose and repeated intravenous dose)". Clinical and Translational Science. 16 (11): 2236–2252. doi:10.1111/cts.13623. PMC 10651655. PMID 37817426.
- ^ Osaka, Hitoshi; Kanazawa, Tetsufumi (December 2023). "Emerging trends in antipsychotic and antidepressant drug development: Targeting nonmonoamine receptors and innovative mechanisms". Psychiatry and Clinical Neurosciences Reports. 2 (4). doi:10.1002/pcn5.157. PMC 11114387. S2CID 265360324.
