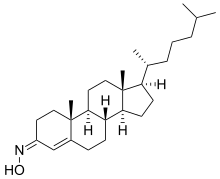
Olesoxime
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C27H45NO |
| Molar mass | 399.663 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Olesoxime (TRO19622) is an experimental drug formerly under development by the now-defunct French company Trophos as a treatment for a range of neuromuscular disorders. It has a cholesterol-like structure and belongs to the cholesterol-oxime family of mitochondrial pore modulators.[1][2]
Research
In preclinical studies, the compound displayed neuroprotective properties by promoting the function and survival of neurons and other cell types under disease-relevant stress conditions. It did so through interactions with two components of the mitochondrial permeability transition pore (mPTP), VDAC and TSPO.[3] In preclinical studies on Huntington's disease, the disease-attenuating effects of olesoxime were attributed to modulating the activity of calcium-dependent proteases called calpains.[4][5]
A 2009–2011 phase 3 clinical trial in amyotrophic lateral sclerosis did not demonstrate an increase in survival.[6] A 2011–2013 trial in spinal muscular atrophy (SMA) indicated that the compound may prevent deterioration of muscle function.[7][8] In 2015, the entire olesoxime programme was purchased by Hoffmann-La Roche for €120 million with a view to developing a treatment for SMA. However, in June 2018, faced with technical and regulatory challenges and competition from a potentially more effective drug nusinersen, Roche halted further development of olesoxime.[9]
References
- ^ Martin LJ (August 2010). "Olesoxime, a cholesterol-like neuroprotectant for the potential treatment of amyotrophic lateral sclerosis". IDrugs. 13 (8): 568–580. PMC 3058503. PMID 20721828.
- ^ "Olesoxime". New Drugs Online Report. UK Medicines Information. Archived from the original on 2016-03-03.
- ^ Bordet T, Buisson B, Michaud M, Drouot C, Galéa P, Delaage P, et al. (August 2007). "Identification and characterization of cholest-4-en-3-one, oxime (TRO19622), a novel drug candidate for amyotrophic lateral sclerosis". The Journal of Pharmacology and Experimental Therapeutics. 322 (2): 709–720. doi:10.1124/jpet.107.123000. PMID 17496168. S2CID 17271734.
- ^ Clemens LE, Weber JJ, Wlodkowski TT, Yu-Taeger L, Michaud M, Calaminus C, et al. (December 2015). "Olesoxime suppresses calpain activation and mutant huntingtin fragmentation in the BACHD rat". Brain. 138 (Pt 12): 3632–3653. doi:10.1093/brain/awv290. PMID 26490331.
- ^ Weber JJ, Ortiz Rios MM, Riess O, Clemens LE, Nguyen HP (2016-01-01). "The calpain-suppressing effects of olesoxime in Huntington's disease". Rare Diseases. 4 (1): e1153778. doi:10.1080/21675511.2016.1153778. PMC 4838320. PMID 27141414.
- ^ "Trophos announces results of phase 3 study of olesoxime in Amyotrophic Lateral Sclerosis". Press Release. Trophos. 2011-12-13. Archived from the original on 2014-02-23.
- ^ "Trophos announces top-line results of pivotal trial of olesoxime in spinal muscular atrophy". Press Release. Trophos. 2014-03-10. Archived from the original on 2014-12-11.
- ^ Bertini E, Dessaud E, Mercuri E, Muntoni F, Kirschner J, Reid C, et al. (July 2017). "Safety and efficacy of olesoxime in patients with type 2 or non-ambulatory type 3 spinal muscular atrophy: a randomised, double-blind, placebo-controlled phase 2 trial". The Lancet. Neurology. 16 (7): 513–522. doi:10.1016/S1474-4422(17)30085-6. hdl:2434/501447. PMID 28460889. S2CID 5842023.
- ^ Taylor, Nick P. (2018-06-01). "Roche scraps €120M SMA drug after hitting 'many difficulties'". www.fiercebiotech.com. Retrieved 2018-06-07.
Further reading
- Rovini A, Carré M, Bordet T, Pruss RM, Braguer D (September 2010). "Olesoxime prevents microtubule-targeting drug neurotoxicity: selective preservation of EB comets in differentiated neuronal cells". Biochemical Pharmacology. 80 (6): 884–894. doi:10.1016/j.bcp.2010.04.018. PMID 20417191.
- Xiao WH, Zheng FY, Bennett GJ, Bordet T, Pruss RM (December 2009). "Olesoxime (cholest-4-en-3-one, oxime): analgesic and neuroprotective effects in a rat model of painful peripheral neuropathy produced by the chemotherapeutic agent, paclitaxel". Pain. 147 (1–3): 202–209. doi:10.1016/j.pain.2009.09.006. PMC 2787910. PMID 19833436.
- Bordet T, Buisson B, Michaud M, Abitbol JL, Marchand F, Grist J, et al. (August 2008). "Specific antinociceptive activity of cholest-4-en-3-one, oxime (TRO19622) in experimental models of painful diabetic and chemotherapy-induced neuropathy". The Journal of Pharmacology and Experimental Therapeutics. 326 (2): 623–632. doi:10.1124/jpet.108.139410. PMID 18492948. S2CID 33726393.
External links
- cholest-4-en-3-one, oxime at the U.S. National Library of Medicine Medical Subject Headings (MeSH)'
