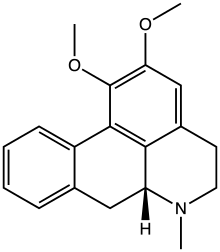
Nuciferine
| |
| Names | |
|---|---|
| IUPAC name 1,2-Dimethoxy-6aβ-aporphine | |
| Systematic IUPAC name (6aR)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline | |
| Other names (R)-1,2-Dimethoxyaporphine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C19H21NO2 | |
| Molar mass | 295.376 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Nuciferine is an alkaloid found within the plants Nymphaea caerulea and Nelumbo nucifera.[1][2]
Preliminary psychopharmacological research in 1978 was unable to conclusively determine the compound's classification regarding dopamine-receptor activity.[3] On one hand, investigative studies found evidence of behavior traditionally associated with dopamine-receptor stimulation: stereotypy, increase in spontaneous motor activity, inhibition of conditioned avoidance response, and an increase in pain sensitivity resulting in an inhibition of morphine analgesia.[3] On the other hand, these early investigative studies also found evidence of behavior traditionally associated with dopamine-receptor blockade: decrease of spontaneous motor activity, chills, catalepsy, trance-like states of consciousness.[3]
Pharmacology
Nuciferine has been reported to have various anti-inflammatory effects, possibly mediated via PPAR delta activation.[4]
According to a newer study from 2016, Nuciferine acts as an antagonist at 5-HT2A, 5-HT2C, and 5-HT2B receptors, an inverse agonist at the 5-HT7 receptor, a partial agonist at D2, D5, and 5-HT6 receptors, and an agonist at 5-HT1A and D4 receptors. Additionally, it inhibits the dopamine transporter (DAT).[5]
In rodent models relating to antipsychotic drug effects, Nuciferine has shown various actions such as blocking head-twitch responses and discriminative stimulus effects of a 5-HT2A agonist, enhancing amphetamine-induced locomotor activity, inhibiting phencyclidine (PCP)-induced locomotor activity, and restoring PCP-induced disruption of pre-pulse inhibition without inducing catalepsy.[5]
Nuciferine may also potentiate morphine analgesia. The median lethal dose in mice is 289 mg/kg. It is structurally related to apomorphine and other aporphine derivatives.[6][7]
See also
References
- ^ Seligman, Sian (2023-01-13). "Blue Lotus Flower: Smoking, Tea & More". DoubleBlind Mag. Retrieved 2023-01-19.
- ^ Farrell, Martilias S.; McCorvy, John D.; Huang, Xi-Ping; Urban, Daniel J.; White, Kate L.; Giguere, Patrick M.; Doak, Allison K.; Bernstein, Alison I.; Stout, Kristen A.; Park, Su Mi; Rodriguiz, Ramona M.; Gray, Bradley W.; Hyatt, William S.; Norwood, Andrew P.; Webster, Kevin A. (2016). "In Vitro and In Vivo Characterization of the Alkaloid Nuciferine". PLOS ONE. 11 (3): e0150602. Bibcode:2016PLoSO..1150602F. doi:10.1371/journal.pone.0150602. ISSN 1932-6203. PMC 4786259. PMID 26963248.
- ^ a b c Bhattacharya, S. K.; Bose, R.; Ghosh, P.; Tripathi, V. J.; Ray, A. B.; Dasgupta, B. (1978-09-15). "Psychopharmacological studies on (--)-nuciferine and its Hofmann degradation product atherosperminine". Psychopharmacology. 59 (1): 29–33. doi:10.1007/bf00428026. ISSN 0033-3158. PMID 100809. S2CID 11847319.
- ^ Zhang, Lina; Gao, Jinghua; Tang, Peng; Chong, Li; Liu, Yue; Liu, Peng; Zhang, Xin; Chen, Li; Hou, Chen (October 2018). "Nuciferine inhibits LPS-induced inflammatory response in BV2 cells by activating PPAR-γ". International Immunopharmacology. 63: 9–13. doi:10.1016/j.intimp.2018.07.015. ISSN 1878-1705. PMID 30056259. S2CID 51894703.
- ^ a b Farrell, Martilias S.; McCorvy, John D.; Huang, Xi-Ping; Urban, Daniel J.; White, Kate L.; Giguere, Patrick M.; Doak, Allison K.; Bernstein, Alison I.; Stout, Kristen A.; Park, Su Mi; Rodriguiz, Ramona M.; Gray, Bradley W.; Hyatt, William S.; Norwood, Andrew P.; Webster, Kevin A. (2016-03-10). "In Vitro and In Vivo Characterization of the Alkaloid Nuciferine". PLOS ONE. 11 (3): e0150602. Bibcode:2016PLoSO..1150602F. doi:10.1371/journal.pone.0150602. ISSN 1932-6203. PMC 4786259. PMID 26963248.
- ^ Bhattacharya SK, Bose R, Ghosh P, Tripathi VJ, Ray AB, Dasgupta B (Sep 1978). "Psychopharmacological studies on (—)-nuciferine and its Hofmann degradation product atherosperminine". Psychopharmacology. 59 (1): 29–33. doi:10.1007/BF00428026. PMID 100809. S2CID 11847319.
- ^ Spess, David L. Errors in Alkaloids of Nelumbo and Nymphaea species, 2011, academia.edu
