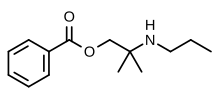
Meprylcaine
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H21NO2 |
| Molar mass | 235.327 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Meprylcaine (also known as Epirocaine and Oracaine) is a local anesthetic with stimulant properties that is structurally related to dimethocaine.[1]
Meprylcaine has a relatively potent inhibitory action on the monoamine transporter and inhibits the reuptake of dopamine, norepinephrine and serotonin.[2][3]
Synthesis
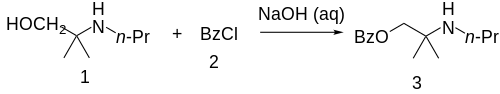
The 2-methyl-2-(propylamino)propan-1-ol [55968-10-0] (1) is treated with base and then with Benzoyl chloride (2), completing the synthesis of Meprycaine (3).
References
- ^ Sato T, Kitayama S, Mitsuhata C, Ikeda T, Morita K, Dohi T (February 2000). "Selective inhibition of monoamine neurotransmitter transporters by synthetic local anesthetics". Naunyn-Schmiedeberg's Archives of Pharmacology. 361 (2): 214–20. doi:10.1007/s002109900184. PMID 10685879. S2CID 1627097.
- ^ Arai S, Morita K, Kitayama S, Kumagai K, Kumagai M, Kihira K, Dohi T (February 2003). "Chronic inhibition of the norepinephrine transporter in the brain participates in seizure sensitization to cocaine and local anesthetics". Brain Research. 964 (1): 83–90. doi:10.1016/S0006-8993(02)04068-4. PMID 12573515. S2CID 16539422.
- ^ Morita K, Hamamoto M, Arai S, Kitayama S, Irifune M, Kawahara M, et al. (September 2005). "Inhibition of serotonin transporters by cocaine and meprylcaine through 5-TH2C receptor stimulation facilitates their seizure activities" (PDF). Brain Research. 1057 (1–2): 153–60. doi:10.1016/j.brainres.2005.07.049. PMID 16125150. S2CID 30437231. Archived from the original (PDF) on 2017-08-17. Retrieved 2019-12-11.
- ^ Reasenberg, Julian R.; Goldberg, Samuel D. (1945). "Esters of β-Alkylaminoethanols". Journal of the American Chemical Society 67 (6): 933–939. doi:10.1021/ja01222a017.
- ^ Julian R Reasenberg, U.S. patent 2,767,207 (1956 to Mizzy Inc).
- ^ Julian R Reasenberg, Samuel D Goldberg, U.S. patent 2,421,129 (1947 to Oradent Chemical Co Inc).
