Dihydrolipoamide dehydrogenase
| DLD | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | DLD, DLDD, DLDH, E3, GCSL, LAD, PHE3, dihydrolipoamide dehydrogenase, Dihydrolipoamide dehydrogenase, OGDC-E3 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 238331; MGI: 107450; HomoloGene: 84; GeneCards: DLD; OMA:DLD - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Dihydrolipoamide dehydrogenase (DLD), also known as dihydrolipoyl dehydrogenase, mitochondrial, is an enzyme that in humans is encoded by the DLD gene.[5][6][7][8] DLD is a flavoprotein enzyme that oxidizes dihydrolipoamide to lipoamide.
Dihydrolipoamide dehydrogenase (DLD) is a mitochondrial enzyme that plays a vital role in energy metabolism in eukaryotes. This enzyme is required for the complete reaction of at least five different multi-enzyme complexes.[9] Additionally, DLD is a flavoenzyme oxidoreductase that contains a reactive disulfide bridge and a FAD cofactor that are directly involved in catalysis. The enzyme associates into tightly bound homodimers required for its enzymatic activity.[10]
Structure
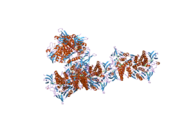
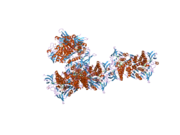
The protein encoded by the DLD gene comes together with another protein to form a dimer in the central metabolic pathway. Several amino acids within the catalytic pocket have been identified as important to DLD function, including R281 and N473.[11][12] Although the overall fold of the human enzyme is similar to that of yeast, the human structure is different in that it has two loops that extend from the general protein structure and into the FAD binding sites when bound the NAD+ molecule, required for catalysis, is not close to the FAD moiety. However, when NADH is bound instead, it is stacked directly op top of the FAD central structure. The current hE3 structures show directly that the disease-causing mutations occur at three locations in the human enzyme: the dimer interface, the active site, and the FAD and NAD(+)-binding sites.[13]
Function
The DLD homodimer functions as the E3 component of the pyruvate, α-ketoglutarate, α-adipate and branched-chain amino acid-dehydrogenase complexes and the glycine cleavage system, all in the mitochondrial matrix. In these complexes, DLD converts dihydrolipoic acid and NAD+ into lipoic acid and NADH.[14] DLD also has diaphorase activity, being able to catalyze the oxidation of NADH to NAD+ by using different electron acceptors such as O2, labile ferric iron, nitric oxide, and ubiquinone.[9] DLD is thought to have a pro-oxidant role by reducing oxygen to a superoxide or ferric to ferrous iron, which then catalyzes production of hydroxyl radicals.[15][16] Diaphorase activity of DLD may have an antioxidant role through its ability to scavenge nitric oxide and to reduce ubiquinone to ubiquinol.[17][18][19] The dihyrolipamide dehydrogenase gene is known to have multiple splice variants.
Moonlighting function
Certain DLD mutations can simultaneously induce the loss of a primary metabolic activity and the gain of a moonlighting proteolytic activity. The moonlighting proteolytic activity of DLD is revealed by conditions that destabilize the DLD homodimer and decrease its DLD activity.[9] Acidification of the mitochondrial matrix, as a result of ischemia-reperfusion injury, can disrupt the quaternary structure of DLD leading to decreased dehydrogenase activity and increased diaphorase activity.[20] The moonlighting proteolytic activity of DLD could also arise under pathological conditions. Proteolytic activity can further complicate the reduction in energy metabolism and an increase in oxidative damage as a result of decreased DLD activity and an increase in diaphorase activity respectively.[19] With its proteolytic function, DLD removes a functionally vital domain from the N-terminus of frataxin, a mitochondrial protein involved in iron metabolism and antioxidant protection.[21][22]
Clinical significance
In humans, mutations in DLD are linked to a severe disorder of infancy with failure to thrive, hypotonia, and metabolic acidosis. [23] DLD deficiency manifests itself in a great degree of variability, which has been attributed to varying effects of different DLD mutations on the stability of the protein and its ability to dimerize or interact with other components of the three α-ketoacid dehydrogenase complexes.[23] With its proteolytic function, DLD causes a deficiency in frataxin, which leads to the neurodegenerative and cardiac disease, Friedreich's ataxia.[24]
Interactive pathway map
| Click on genes, proteins and metabolites below to link to respective articles. [§ 1] TCACycle_WP78 edit
|
Click on genes, proteins and metabolites below to link to respective articles.[§ 1] Glycolysis and Gluconeogenesis edit
|
Enzyme regulation
This protein may use the morpheein model of allosteric regulation.[25]
See also
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000091140 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000020664 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Entrez Gene: dihydrolipoamide dehydrogenase".
- ^ Otulakowski G, Robinson BH (December 1987). "Isolation and sequence determination of cDNA clones for porcine and human lipoamide dehydrogenase. Homology to other disulfide oxidoreductases". The Journal of Biological Chemistry. 262 (36): 17313–8. doi:10.1016/S0021-9258(18)45379-3. PMID 3693355.
- ^ Pons G, Raefsky-Estrin C, Carothers DJ, Pepin RA, Javed AA, Jesse BW, et al. (March 1988). "Cloning and cDNA sequence of the dihydrolipoamide dehydrogenase component human alpha-ketoacid dehydrogenase complexes". Proceedings of the National Academy of Sciences of the United States of America. 85 (5): 1422–6. Bibcode:1988PNAS...85.1422P. doi:10.1073/pnas.85.5.1422. PMC 279783. PMID 3278312.
- ^ Scherer SW, Otulakowski G, Robinson BH, Tsui LC (1991). "Localization of the human dihydrolipoamide dehydrogenase gene (DLD) to 7q31----q32". Cytogenetics and Cell Genetics. 56 (3–4): 176–7. doi:10.1159/000133081. hdl:10722/42531. PMID 2055113.
- ^ a b c Babady NE, Pang YP, Elpeleg O, Isaya G (April 2007). "Cryptic proteolytic activity of dihydrolipoamide dehydrogenase". Proceedings of the National Academy of Sciences of the United States of America. 104 (15): 6158–63. Bibcode:2007PNAS..104.6158B. doi:10.1073/pnas.0610618104. PMC 1851069. PMID 17404228.
- ^ Ciszak EM, Makal A, Hong YS, Vettaikkorumakankauv AK, Korotchkina LG, Patel MS (January 2006). "How dihydrolipoamide dehydrogenase-binding protein binds dihydrolipoamide dehydrogenase in the human pyruvate dehydrogenase complex". The Journal of Biological Chemistry. 281 (1): 648–55. doi:10.1074/jbc.M507850200. PMID 16263718.
- ^ Kim H (March 2005). "Asparagine-473 residue is important to the efficient function of human dihydrolipoamide dehydrogenase". Journal of Biochemistry and Molecular Biology. 38 (2): 248–52. doi:10.5483/bmbrep.2005.38.2.248. PMID 15826505.
- ^ Wang YC, Wang ST, Li C, Chen LY, Liu WH, Chen PR, et al. (January 2008). "The role of amino acids T148 and R281 in human dihydrolipoamide dehydrogenase". Journal of Biomedical Science. 15 (1): 37–46. doi:10.1007/s11373-007-9208-9. PMID 17960497.
- ^ Brautigam CA, Chuang JL, Tomchick DR, Machius M, Chuang DT (July 2005). "Crystal structure of human dihydrolipoamide dehydrogenase: NAD+/NADH binding and the structural basis of disease-causing mutations". Journal of Molecular Biology. 350 (3): 543–52. doi:10.1016/j.jmb.2005.05.014. PMID 15946682.
- ^ Carothers DJ, Pons G, Patel MS (February 1989). "Dihydrolipoamide dehydrogenase: functional similarities and divergent evolution of the pyridine nucleotide-disulfide oxidoreductases". Archives of Biochemistry and Biophysics. 268 (2): 409–25. doi:10.1016/0003-9861(89)90309-3. PMID 2643922.
- ^ Petrat F, Paluch S, Dogruöz E, Dörfler P, Kirsch M, Korth HG, et al. (November 2003). "Reduction of Fe(III) ions complexed to physiological ligands by lipoyl dehydrogenase and other flavoenzymes in vitro: implications for an enzymatic reduction of Fe(III) ions of the labile iron pool". The Journal of Biological Chemistry. 278 (47): 46403–13. doi:10.1074/jbc.M305291200. PMID 12963736.
- ^ Yoneyama K, Shibata R, Igarashi A, Kojima S, Kodani Y, Nagata K, et al. (September 2014). "Proteomic identification of dihydrolipoamide dehydrogenase as a target of autoantibodies in patients with endometrial cancer". Anticancer Research. 34 (9): 5021–7. PMID 25202086.
- ^ Igamberdiev AU, Bykova NV, Ens W, Hill RD (June 2004). "Dihydrolipoamide dehydrogenase from porcine heart catalyzes NADH-dependent scavenging of nitric oxide". FEBS Letters. 568 (1–3): 146–50. Bibcode:2004FEBSL.568..146I. doi:10.1016/j.febslet.2004.05.024. PMID 15196936. S2CID 20180110.
- ^ Olsson JM, Xia L, Eriksson LC, Björnstedt M (April 1999). "Ubiquinone is reduced by lipoamide dehydrogenase and this reaction is potently stimulated by zinc". FEBS Letters. 448 (1): 190–2. Bibcode:1999FEBSL.448..190O. doi:10.1016/s0014-5793(99)00363-4. PMID 10217438. S2CID 34370150.
- ^ a b Xia L, Björnstedt M, Nordman T, Eriksson LC, Olsson JM (March 2001). "Reduction of ubiquinone by lipoamide dehydrogenase. An antioxidant regenerating pathway". European Journal of Biochemistry. 268 (5): 1486–90. doi:10.1046/j.1432-1327.2001.02013.x. PMID 11231302.
- ^ Klyachko NL, Shchedrina VA, Efimov AV, Kazakov SV, Gazaryan IG, Kristal BS, Brown AM (April 2005). "pH-dependent substrate preference of pig heart lipoamide dehydrogenase varies with oligomeric state: response to mitochondrial matrix acidification". The Journal of Biological Chemistry. 280 (16): 16106–14. doi:10.1074/jbc.M414285200. PMID 15710613.
- ^ Al-Karadaghi S, Franco R, Hansson M, Shelnutt JA, Isaya G, Ferreira GC (March 2006). "Chelatases: distort to select?". Trends in Biochemical Sciences. 31 (3): 135–42. doi:10.1016/j.tibs.2006.01.001. PMC 2997100. PMID 16469498.
- ^ O'Neill HA, Gakh O, Park S, Cui J, Mooney SM, Sampson M, et al. (January 2005). "Assembly of human frataxin is a mechanism for detoxifying redox-active iron". Biochemistry. 44 (2): 537–45. doi:10.1021/bi048459j. PMID 15641778.
- ^ a b Quinonez SC, Thoene JG (9 July 2020). "Dihydrolipoamide Dehydrogenase Deficiency". In Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJ, Mirzaa G, Amemiya A (eds.). GeneReviews. University of Washington, Seattle. PMID 25032271.
- ^ Ambrus A, Adam-Vizi V (July 2018). "Human dihydrolipoamide dehydrogenase (E3) deficiency: Novel insights into the structural basis and molecular pathomechanism" (PDF). Neurochemistry International. 117: 5–14. doi:10.1016/j.neuint.2017.05.018. PMID 28579060. S2CID 38777180.
- ^ Selwood T, Jaffe EK (March 2012). "Dynamic dissociating homo-oligomers and the control of protein function". Archives of Biochemistry and Biophysics. 519 (2): 131–43. doi:10.1016/j.abb.2011.11.020. PMC 3298769. PMID 22182754.
Further reading
- Silverberg MS, Cho JH, Rioux JD, McGovern DP, Wu J, Annese V, et al. (February 2009). "Ulcerative colitis-risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study". Nature Genetics. 41 (2): 216–20. doi:10.1038/ng.275. PMC 2652837. PMID 19122664.
- Brautigam CA, Chuang JL, Tomchick DR, Machius M, Chuang DT (July 2005). "Crystal structure of human dihydrolipoamide dehydrogenase: NAD+/NADH binding and the structural basis of disease-causing mutations". Journal of Molecular Biology. 350 (3): 543–52. doi:10.1016/j.jmb.2005.05.014. PMID 15946682.
- Reed LJ, Hackert ML (June 1990). "Structure-function relationships in dihydrolipoamide acyltransferases". The Journal of Biological Chemistry. 265 (16): 8971–4. doi:10.1016/S0021-9258(19)38795-2. PMID 2188967.
- Ciszak EM, Makal A, Hong YS, Vettaikkorumakankauv AK, Korotchkina LG, Patel MS (January 2006). "How dihydrolipoamide dehydrogenase-binding protein binds dihydrolipoamide dehydrogenase in the human pyruvate dehydrogenase complex". The Journal of Biological Chemistry. 281 (1): 648–55. doi:10.1074/jbc.M507850200. PMID 16263718.
- Asano K, Matsushita T, Umeno J, Hosono N, Takahashi A, Kawaguchi T, et al. (December 2009). "A genome-wide association study identifies three new susceptibility loci for ulcerative colitis in the Japanese population". Nature Genetics. 41 (12): 1325–9. doi:10.1038/ng.482. PMID 19915573. S2CID 20507558.
- Odièvre MH, Chretien D, Munnich A, Robinson BH, Dumoulin R, Masmoudi S, et al. (March 2005). "A novel mutation in the dihydrolipoamide dehydrogenase E3 subunit gene (DLD) resulting in an atypical form of alpha-ketoglutarate dehydrogenase deficiency". Human Mutation. 25 (3): 323–4. doi:10.1002/humu.9319. PMID 15712224. S2CID 19929944.
- Brautigam CA, Wynn RM, Chuang JL, Machius M, Tomchick DR, Chuang DT (March 2006). "Structural insight into interactions between dihydrolipoamide dehydrogenase (E3) and E3 binding protein of human pyruvate dehydrogenase complex". Structure. 14 (3): 611–21. doi:10.1016/j.str.2006.01.001. PMC 2879633. PMID 16442803.
- Kim H (March 2006). "Activity of human dihydrolipoamide dehydrogenase is largely reduced by mutation at isoleucine-51 to alanine". Journal of Biochemistry and Molecular Biology. 39 (2): 223–7. doi:10.5483/bmbrep.2006.39.2.223. PMID 16584639.
- Sugden MC, Holness MJ (May 2003). "Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs". American Journal of Physiology. Endocrinology and Metabolism. 284 (5): E855-62. doi:10.1152/ajpendo.00526.2002. PMID 12676647.
- Wang YC, Wang ST, Li C, Chen LY, Liu WH, Chen PR, et al. (January 2008). "The role of amino acids T148 and R281 in human dihydrolipoamide dehydrogenase". Journal of Biomedical Science. 15 (1): 37–46. doi:10.1007/s11373-007-9208-9. PMID 17960497.
- Brown AM, Gordon D, Lee H, Caudy M, Hardy J, Haroutunian V, Blass JP (November 2004). "Association of the dihydrolipoamide dehydrogenase gene with Alzheimer's disease in an Ashkenazi Jewish population". American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 131B (1): 60–6. doi:10.1002/ajmg.b.30008. PMID 15389771. S2CID 26098296.
- Wang YC, Wang ST, Li C, Liu WH, Chen PR, Chen LY, Liu TC (March 2007). "The role of N286 and D320 in the reaction mechanism of human dihydrolipoamide dehydrogenase (E3) center domain". Journal of Biomedical Science. 14 (2): 203–10. doi:10.1007/s11373-006-9136-0. PMID 17171578.
- Foster LJ, Rudich A, Talior I, Patel N, Huang X, Furtado LM, et al. (January 2006). "Insulin-dependent interactions of proteins with GLUT4 revealed through stable isotope labeling by amino acids in cell culture (SILAC)". Journal of Proteome Research. 5 (1): 64–75. doi:10.1021/pr0502626. PMID 16396496.
- Kim H (March 2005). "Asparagine-473 residue is important to the efficient function of human dihydrolipoamide dehydrogenase". Journal of Biochemistry and Molecular Biology. 38 (2): 248–52. doi:10.5483/bmbrep.2005.38.2.248. PMID 15826505.
- Hiromasa Y, Fujisawa T, Aso Y, Roche TE (February 2004). "Organization of the cores of the mammalian pyruvate dehydrogenase complex formed by E2 and E2 plus the E3-binding protein and their capacities to bind the E1 and E3 components". The Journal of Biological Chemistry. 279 (8): 6921–33. doi:10.1074/jbc.M308172200. PMID 14638692.
- Wynn RM, Kato M, Machius M, Chuang JL, Li J, Tomchick DR, Chuang DT (December 2004). "Molecular mechanism for regulation of the human mitochondrial branched-chain alpha-ketoacid dehydrogenase complex by phosphorylation". Structure. 12 (12): 2185–96. doi:10.1016/j.str.2004.09.013. PMID 15576032.
- Martins-de-Souza D, Gattaz WF, Schmitt A, Novello JC, Marangoni S, Turck CW, Dias-Neto E (April 2009). "Proteome analysis of schizophrenia patients Wernicke's area reveals an energy metabolism dysregulation". BMC Psychiatry. 9: 17. doi:10.1186/1471-244X-9-17. PMC 2684104. PMID 19405953.
External links
- Dihydrolipoamide+dehydrogenase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
This article incorporates text from the United States National Library of Medicine, which is in the public domain.