Leghemoglobin
| Leghaemoglobin, iron-binding site | |
|---|---|
| Identifiers | |
| Symbol | Leghaemoglobin_Fe_BS |
| InterPro | IPR019824 |
| PROSITE | PS00208 |
Leghemoglobin (also leghaemoglobin or legoglobin) is an oxygen-carrying phytoglobin found in the nitrogen-fixing root nodules of leguminous plants. It is produced by these plants in response to the roots being colonized by nitrogen-fixing bacteria, termed rhizobia, as part of the symbiotic interaction between plant and bacterium: roots not colonized by Rhizobium do not synthesise leghemoglobin. Leghemoglobin has close chemical and structural similarities to hemoglobin, and, like hemoglobin, is red in colour. It was originally thought that the heme prosthetic group for plant leghemoglobin was provided by the bacterial symbiont within symbiotic root nodules.[1][2] However, subsequent work shows that the plant host strongly expresses heme biosynthesis genes within nodules, and that activation of those genes correlates with leghemoglobin gene expression in developing nodules.[3][4][5][6][7][8][9][10]
In plants colonised by Rhizobium, such as alfalfa or soybeans, the presence of oxygen in the root nodules would reduce the activity of the oxygen-sensitive nitrogenase, which is an enzyme responsible for the fixation of atmospheric nitrogen. Leghemoglobin is shown to buffer the concentration of free oxygen in the cytoplasm of infected plant cells to ensure the proper function of root nodules. That being said, nitrogen fixation is an extremely energetically costly process, so aerobic respiration, which necessitates high oxygen concentration, is necessary in the cells of the root nodule.[11] Leghemoglobin maintains a free oxygen concentration that is low enough to allow nitrogenase to function, but a high enough total oxygen concentration (free and bound to leghemoglobin) for aerobic respiration.
Leghemoglobin falls into the class of symbiotic globins, which also include the root nodules globins of actinorhizal plants such as Casuarina. The Casuarina symbiotic globin is intermediate between leghemoglobin and nonsymbiotic phytoglobin-2.[12][13]
Structure
Leghemoglobins are monomeric proteins with a mass around 16 kDa, and are structurally similar to myoglobin.[14] One leghemoglobin protein consists of a heme bound to an iron, and one polypeptide chain (the globin).[14] Similar to myoglobin and hemoglobin, the iron of heme is found in its ferrous state in vivo, and is the moiety that binds oxygen.[14] Despite similarities in the mechanism of oxygen binding between leghemoglobin and animal hemoglobin, and the fact that leghemoglobin and animal hemoglobin evolved from a common ancestor, there is dissimilarity in amino acid sequence between these proteins at about 80% of positions.[14]
Oxygen binding affinities of leghemoglobins are between 11 and 24 times higher than oxygen binding affinities of sperm whale myoglobin.[15] Differences in the affinities are due to differential rates of association between the two types of proteins.[15] One explanation of this phenomenon is that in myoglobin, a bound water molecule is stabilized in a pocket surrounding the heme group. This water group must be displaced in order for oxygen to bind. No such water is bound in the analogous pocket of leghemoglobin, so it is easier for an oxygen molecule to approach the leghemoglobin heme.[14] Leghemoglobin has a slow oxygen dissociation rate, similar to myoglobin.[16] Like myoglobin and hemoglobin, leghemoglobin has a high affinity for carbon monoxide.[16]
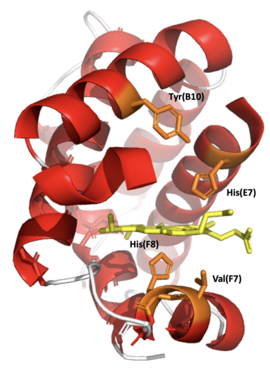
In the primary structure of Leghemoglobin A in soybeans, a valine(F7) is found in place where a serine(F7) is in Myoglobin. Without a hydrogen bond fixing the orientation of the proximal histidine side chain the imidazole ring can occupy a staggered conformation between pyrrole nitrogen atoms and can readily move upward to the heme plane. This greatly increases the reactivity of the iron atom and oxygen affinity. In Leghemoglobin A the distal histidine side chain is also rotated away from the bound ligand by formation of a hydrogen bond with Tyrosine.[17]
Heme groups are the same in all known leghemoglobins, but the amino acid sequence of the globin differs slightly depending on bacterial strain and legume species.[14] Even within one leguminous plant, multiple isoforms of leghemoglobins can exist. These often differ in oxygen affinity, and help meet the needs of a cell in a particular environment within the nodule.[18]
Debate on principal function
Results of a 1995 study suggested that the low free oxygen concentration in root nodule cells is actually due to the low oxygen permeability of root nodule cells.[19] It follows that the main purpose of leghemoglobin is to scavenge the limited free oxygen in the cell and deliver it to mitochondria for respiration. But, scientists of a later 2005 article suggest that leghemoglobin is responsible both for buffering oxygen concentration, and for delivery of oxygen to mitochondria.[20] Their leghemoglobin knockout studies showed that leghemoglobin actually does significantly decrease the free oxygen concentration in root nodule cells, and that nitrogenase expression was eliminated in leghemoglobin knockout mutants, assumably due to the degradation of nitrogenase with high free oxygen concentration. Their study also showed a higher ATP/ADP ratio in wild-type root nodule cells with active leghemoglobin, suggesting that leghemoglobin also assists with delivery of oxygen for respiration.
Plants contain both symbiotic and nonsymbiotic hemoglobins. Symbiotic hemoglobins are thought to be important for symbiotic nitrogen fixation (SNF). In legume, SNF takes place in specialized organs called nodules which contain bacteroids, or nitrogen fixing rhizobia. The induction of nodule-specific plant genes, which include those that encode for symbiotic leghemoglobins (Lb), accompany nodule development. Leghemoglobins accumulate to millimolar concentrations in the cytoplasm of infected plant cells prior to nitrogen fixation to buffer free oxygen in the nanomolar range, which can avoid inactivation of oxygen-labile nitrogenase while keeping a high enough oxygen flux for respiration in the cell. The leghemoglobins are required for SNF but are not required for plant growth and development in the presence of an external source of fixed nitrogen. Leghemoglobins make the essential contribution of establishing low free-oxygen concentrations while keep a high energy status in cells. These are the conditions necessary for effective SNF.[20]
Other plant hemoglobins
Globins have since been identified as a protein common to many plant taxa, not restricted to symbiotic ones. In light of this discovery, it has been proposed that the term phytoglobins be used for referring to plant globins in general.[12]
Phytoglobins can be divided into two clades. The 3/3-fold type contains Classes I and II of angiosperm phytoglobins, and is the one common to all eukaryotes (HGT of a bacterial flavohemoglobin). The leghemoglobin sensu stricto is a class II phytoglobin. The 2/2-fold "TrHb2" type contains class III in angiosperm nomenclature, and appears to be acquired from Chloroflexota (formerly Chloroflexi) by the ancestor of land plants.[12]
Commercial use
Impossible Foods asked the American FDA for their approval to use recombinant soy leghemoglobin in meat alternatives as an analog of meat-derived hemoglobin.[21][22] Approval from the FDA came in July 2019,[23] was challenged,[a] and later upheld, on May 3, 2021, by a San Francisco federal appeals court.[24][25] It is currently being used in their products to mimic the color, taste, and texture of meat.[26]
See also
References
- ^ Nadler KD, Avissar YJ (September 1977). "Heme Synthesis in Soybean Root Nodules: I. On the Role of Bacteroid delta-Aminolevulinic Acid Synthase and delta-Aminolevulinic Acid Dehydrase in the Synthesis of the Heme of Leghemoglobin". Plant Physiology. 60 (3): 433–6. doi:10.1104/pp.60.3.433. PMC 542631. PMID 16660108.
- ^ O'Brian MR, Kirshbom PM, Maier RJ (December 1987). "Bacterial heme synthesis is required for expression of the leghemoglobin holoprotein but not the apoprotein in soybean root nodules". Proceedings of the National Academy of Sciences of the United States of America. 84 (23): 8390–3. Bibcode:1987PNAS...84.8390O. doi:10.1073/pnas.84.23.8390. PMC 299548. PMID 3479799.
- ^ Sangwan I, O'Brian MR (March 1991). "Evidence for an inter-organismic heme biosynthetic pathway in symbiotic soybean root nodules". Science. 251 (4998): 1220–2. Bibcode:1991Sci...251.1220S. doi:10.1126/science.251.4998.1220. PMID 17799282. S2CID 11471787.
- ^ Sangwan I, O'Brian MR (March 1992). "Characterization of delta-Aminolevulinic Acid Formation in Soybean Root Nodules". Plant Physiology. 98 (3): 1074–9. doi:10.1104/pp.98.3.1074. PMC 1080310. PMID 16668729.
- ^ Sangwan I, O'Brian MR (July 1993). "Expression of the soybean (Glycine max) glutamate 1-semialdehyde aminotransferase gene in symbiotic root nodules". Plant Physiology. 102 (3): 829–34. doi:10.1104/pp.102.3.829. PMC 158853. PMID 8278535.
- ^ Madsen O, Sandal L, Sandal NN, Marcker KA (October 1993). "A soybean coproporphyrinogen oxidase gene is highly expressed in root nodules". Plant Molecular Biology. 23 (1): 35–43. doi:10.1007/BF00021417. PMID 8219054. S2CID 23011457.
- ^ Kaczor CM, Smith MW, Sangwan I, O'Brian MR (April 1994). "Plant delta-aminolevulinic acid dehydratase. Expression in soybean root nodules and evidence for a bacterial lineage of the Alad gene". Plant Physiology. 104 (4): 1411–7. doi:10.1104/pp.104.4.1411. PMC 159307. PMID 8016269.
- ^ Frustaci JM, Sangwan I, O'Brian MR (March 1995). "gsa1 is a universal tetrapyrrole synthesis gene in soybean and is regulated by a GAGA element". The Journal of Biological Chemistry. 270 (13): 7387–93. doi:10.1074/jbc.270.13.7387. PMID 7706283.
- ^ Santana MA, Pihakaski-Maunsbach K, Sandal N, Marcker KA, Smith AG (April 1998). "Evidence that the plant host synthesizes the heme moiety of leghemoglobin in root nodules". Plant Physiology. 116 (4): 1259–69. doi:10.1104/pp.116.4.1259. PMC 35032. PMID 9536042.
- ^ Sangwan I, O'Brian MR (February 1999). "Expression of a soybean gene encoding the tetrapyrrole-synthesis enzyme glutamyl-tRNA reductase in symbiotic root nodules". Plant Physiology. 119 (2): 593–8. doi:10.1104/pp.119.2.593. PMC 32136. PMID 9952455.
- ^ Berg, J.; Tymoczko, J.; Gatto Jr., G.; Stryer, L. (2015). Biochemistry (8th ed.). W.H. & Freeman Company.
- ^ a b c Becana, Manuel; Yruela, Inmaculada; Sarath, Gautam; Catalán, Pilar; Hargrove, Mark S. (September 2020). "Plant hemoglobins: a journey from unicellular green algae to vascular plants". New Phytologist. 227 (6): 1618–1635. doi:10.1111/nph.16444. hdl:10261/219101. PMID 31960995.
- ^ Hill R, Hargrove MS, Arredondo-Peter R (2016). "Phytoglobin: a novel nomenclature for plant globins accepted by the globin community at the 2014 XVIII conference on Oxygen-Binding and Sensing Proteins". F1000Research. 5: 212. doi:10.12688/f1000research.8133.1. PMC 4792203. PMID 26998237.
- ^ a b c d e f Singh S., Varma A. (2017) Structure, Function, and Estimation of Leghemoglobin. In: Hansen A., Choudhary D., Agrawal P., Varma A. (eds) Rhizobium Biology and Biotechnology. Soil Biology, vol 50. Springer, Cham
- ^ a b Harutyunyan EH, Safonova TN, Kuranova IP, Popov AN, Teplyakov AV, Obmolova GV, Rusakov AA, Vainshtein BK, Dodson GG, Wilson JC. "The structure of deoxy- and oxy-leghaemoglobin from lupin".
{{cite journal}}: Cite journal requires|journal=(help)[full citation needed] - ^ a b Wittenberg JB, Appleby CA, Wittenberg BA (January 1972). "The Kinetics of the Reactions of Leghemoglobin with Oxygen and Carbon Monoxide". Journal of Biological Chemistry. 247 (2): 527–531. doi:10.1016/S0021-9258(19)45734-7. PMID 4333266.
- ^ Smagghe, Benoit J.; Hoy, Julie A.; Percifield, Ryan; Kundu, Suman; Hargrove, Mark S.; Sarath, Gautam; Hilbert, Jean-Louis; Watts, Richard A.; Dennis, Elizabeth S.; Peacock, W. James; Dewilde, Sylvia; Moens, Luc; Blouin, George C.; Olson, John S.; Appleby, Cyril A. (December 2009). "Review: correlations between oxygen affinity and sequence classifications of plant hemoglobins". Biopolymers. 91 (12): 1083–1096. doi:10.1002/bip.21256. ISSN 0006-3525. PMID 19441024. S2CID 1891302.
- ^ Kawashima K, Suganuma N, Tamaoki M, Kouchi H (2001). "Two types of pea leghemoglobin genes showing different O2-binding affinities and distinct patterns of spatial expression in nodules". Plant Physiol. 125 (2): 641–651. doi:10.1104/pp.125.2.641. PMC 64866. PMID 11161022.
- ^ Ludwig RA, de Vries GE (1986). "Biochemical physiology of Rhizobium dinitrogen fixation". In Broughton WJ, Pühler S (eds.). Nitrogen Fixation, Vol. 4: Molecular Biology. Oxford, UK: Clarendon University Press. pp. 50–69. ISBN 978-0-19-854575-0.
- ^ a b Ott, Thomas; van Dongen, Joost T.; Günther, Catrin; Krusell, Lene; Desbrosses, Guilhem; Vigeolas, Helene; Bock, Vivien; Czechowski, Tomasz; Geigenberger, Peter; Udvardi, Michael K. (2005-03-29). "Symbiotic leghemoglobins are crucial for nitrogen fixation in legume root nodules but not for general plant growth and development". Current Biology. 15 (6): 531–535. Bibcode:2005CBio...15..531O. doi:10.1016/j.cub.2005.01.042. ISSN 0960-9822. PMID 15797021.
- ^ "GRAS Notice 540". www.accessdata.fda.gov. Archived from the original on 2017-06-30. Retrieved 2018-01-21.
- ^ "GRAS Notice 737". www.accessdata.fda.gov. Retrieved 2018-08-22.[permanent dead link]
- ^ "Beyond Meat's competitor Impossible Foods plans to launch in grocery stores in September after getting FDA approval". CNBC. 31 July 2019. Retrieved 31 July 2019.
- ^ Justine Calma (May 3, 2021). "Impossible Foods clears legal battle over the ingredient that makes its meat 'bleed'". theverge.com. Archived from the original on 2021-05-07.
- ^ Sally Ho (May 6, 2021). "Impossible Foods Wins Legal Battle Over Heme Ingredient Powering 'Bleeding' Plant-Based Burger". greenqueen.com.hk. Archived from the original on 2021-05-06.
- ^ Bandoim, L. (December 20, 2019). "What The FDA's Decision About Soy Leghemoglobin Means For Impossible Burger". Forbes. Retrieved March 4, 2020.
Notes
- ^ filed by the non-profit advocacy organization Center for Food Safety
Further reading
- Virtanen AI (1948). "Biological nitrogen fixation". Annual Review of Microbiology. 2 (1): 485–506. doi:10.1146/annurev.mi.02.100148.002413. PMID 18122253.
- Taiz, L.; Zeiger, E. (2006). Plant Physiology Online (3rd ed.). Sunderland, MA: Sinauwr Associates, Inc. p. 269. ISBN 978-0-87893-856-8. Archived from the original on 2008-05-09. Retrieved 2008-05-03.
- Impossible Burger’s ‘Secret Sauce’ Highlights Challenges of Food Tech
- Updates FDA Announces Effective Date for Final Rule Adding Soy Leghemoglobin to List of Color Additives Exempt from Certification
