Iron(II) phosphate
| |

| |
| Names | |
|---|---|
| IUPAC name Iron(II) phosphate | |
| Other names Ferrous phosphate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.035.456 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
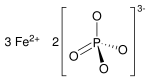
| Fe3(PO4)2 | |
| Appearance | brown powder |
| Density | 2.61 g/cm3 (octahydrate) |
| Melting point | 180 °C (356 °F; 453 K) (octahydrate) decomposes[1] |
| insoluble | |
| Structure | |
| monoclinic (octahydrate) | |
| C 2/m | |
a = 10.086 (octahydrate), b = 13.441 (octahydrate), c = 4.703 (octahydrate) α = 90°, β = 104.27°, γ = 90° | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P280, P304+P340, P305+P351+P338, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Iron(II) phosphate, also ferrous phosphate,[3] Fe3(PO4)2, is an iron salt of phosphoric acid.
Natural occurrences
The mineral vivianite is a naturally occurring form of hydrated iron(II) phosphate.
Production
It can be formed by the reaction of ferrous hydroxide with phosphoric acid to produce hydrated iron(II) phosphate.
See also
References
- ^ "iron(II) phosphate octahydrate". chemister.ru. Retrieved 2 July 2014.
- ^ "Safety Data Sheet". fishersci.com. Retrieved 12 August 2023.
- ^ "Iron(II) Phosphate". EndMemo.com. Retrieved 22 January 2016.
External links
![]() Media related to Iron(II) phosphate at Wikimedia Commons
Media related to Iron(II) phosphate at Wikimedia Commons

