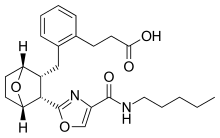
Ifetroban
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H32N2O5 |
| Molar mass | 440.540 g·mol−1 |
| 3D model (JSmol) | |
| |
Ifetroban is a potent and selective thromboxane receptor antagonist.[1] It has been studied in animal models for the treatment of cancer metastasis,[2] myocardial ischemia, hypertension, stroke, thrombosis, cardiomyopathy,[3] and for its effects on platelets.[4][5] Clinical trials are evaluating the therapeutic safety and efficacy of oral ifetroban capsules for the treatment of cancer metastasis,[6] cardiovascular disease,[7] aspirin exacerbated respiratory disease,[8][9][10] systemic sclerosis,[11] and Duchenne muscular dystrophy.[12]
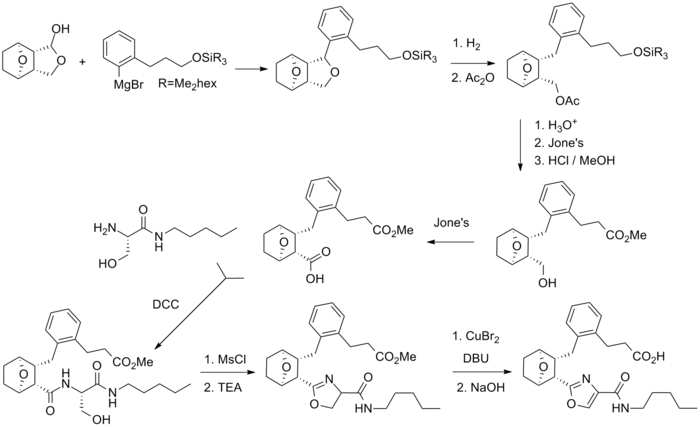
Synthesis
References
- ^ Rosenfeld L, Grover GJ, Stier CT (2006). "Ifetroban sodium: an effective TxA2/PGH2 receptor antagonist". Cardiovascular Drug Reviews. 19 (2): 97–115. doi:10.1111/j.1527-3466.2001.tb00058.x. PMID 11484065.
- ^ Werfel TA, Hicks DJ, Rahman B, Bendeman WE, Duvernay MT, Maeng JG, et al. (December 2020). "Repurposing of a Thromboxane Receptor Inhibitor Based on a Novel Role in Metastasis Identified by Phenome-Wide Association Study". Molecular Cancer Therapeutics. 19 (12): 2454–2464. doi:10.1158/1535-7163.MCT-19-1106. PMID 33033174. S2CID 222237252.
- ^ West JD, Voss BM, Pavliv L, de Caestecker M, Hemnes AR, Carrier EJ (June 2016). "Antagonism of the thromboxane-prostanoid receptor is cardioprotective against right ventricular pressure overload". Pulmonary Circulation. 6 (2): 211–223. doi:10.1086/686140. PMC 4869926. PMID 27252848.
- ^ Dockens RC, Santone KS, Mitroka JG, Morrison RA, Jemal M, Greene DS, Barbhaiya RH (August 2000). "Disposition of radiolabeled ifetroban in rats, dogs, monkeys, and humans" (PDF). Drug Metabolism and Disposition. 28 (8): 973–980. PMID 10901709.
- ^ West JD, Galindo CL, Kim K, Shin JJ, Atkinson JB, Macias-Perez I, et al. (November 2019). "Antagonism of the Thromboxane-Prostanoid Receptor as a Potential Therapy for Cardiomyopathy of Muscular Dystrophy". Journal of the American Heart Association. 8 (21): e011902. doi:10.1161/JAHA.118.011902. PMC 6898850. PMID 31662020.
- ^ Clinical trial number NCT03694249 for "A Pilot Trial of Ifetroban, A Thromboxane A2 Receptor Antagonist, in Patients With Malignant Solid Tumors at High Risk of Metastatic Recurrence" at ClinicalTrials.gov
- ^ Clinical trial number NCT03962855 for "The Thromboxane Receptor Antagonist to Blunt the Effects of Non-Platelet Thromboxane Generation and Improve Endothelial Cell Function (TRAP) Trial" at ClinicalTrials.gov
- ^ Clinical trial number NCT02216357 for "A Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial to Determine the Safety of Oral Ifetroban in Patients With a History of Aspirin Exacerbated Respiratory Disease" at ClinicalTrials.gov
- ^ Clinical trial number NCT03326063 for "Therapeutic Control of Aspirin-Exacerbated Respiratory Disease" at ClinicalTrials.gov
- ^ Clinical trial number NCT03028350 for "A Phase 2 Multicenter, Double-blind, Randomized, Placebo-Controlled Trial to Evaluate Oral Ifetroban in Subjects With Symptomatic Aspirin Exacerbated Respiratory Disease (AERD)" at ClinicalTrials.gov
- ^ Clinical trial number NCT02682511 for "A Phase 2 Multicenter, Randomized, Double-blind, Placebo-controlled Study to Assess the Safety and Efficacy of Ifetroban in Patients With Diffuse Cutaneous Systemic Sclerosis (SSc) or SSc-associated Pulmonary Arterial Hypertension (SSc-PAH) " at ClinicalTrials.gov
- ^ Clinical trial number NCT03340675 for "A Randomized, Double-Blind, Placebo-Controlled, Multiple Dose Study With an Open-Label Extension to Determine the Safety, Pharmacokinetics and Efficacy of Oral Ifetroban in Subjects With Duchenne Muscular Dystrophy " at ClinicalTrials.gov
