Glossary of tunicate anatomy

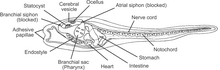
Tunicates (subphylum Tunicata) are a group of filter-feeding marine invertebrates.[1] As chordates, they have been proposed to be the closest relatives of vertebrates.[2] Tunicates are divided into three classes: the sessile Ascidiacea, and the free-swimming Thaliacea and Appendicularia.[1] Some anatomical features are shared between classes, while others are specific to a lineage or to a life stage.
- adhesive organ
- 1. An organ present at the anterior end of ascidian larvae, serving to attach the larva to the substrate during its metamorphosis. It is usually made of three papillae.[3]
- 2. The individual papillae.[4]
- atrium
- atrial pore
- atrial siphon
-
Opening through which water exits the branchial basket in ascidians.[5]
- blastozooid
- Sexual generation in the life cycle of salps. They grow from the parent oozooid before budding off and sexually reproducing, with internal fertilization. In most species, females are viviparous and grow a single oozooid.[6]
- branchial basket
-
Main pumping and filtering cavity of the tunicate body. It is lined with stigmata through which water is filtered. In ascidians, the branchial basket can be flat, or folded in various arrangements.[7]
- brooding
- Method of reproduction in ascidians, where zygotes are incubated inside the body of the individual.[8] In some species, a dedicated brood pouch is present.[9]
- budding
- Method of reproduction in colonial tunicates, where new zooids, or "buds", grow from the body of existing zooids or from stolons.[10]
- cadophore
- Stolon-like posterior extension of the doliolid nurse comprising three pairs of rows from which phorozooids and trophozooids develop.[11][12]
- cerebral ganglion
- coronal organ
- Sensory organ present in the oral aperture of ascidians and most thaliaceans (except salps). Made of a row of secondary sensory cells (hair cells), it monitors the flow of incoming water and prevents large particles from entering.[13] It has been suggested to be homologous to the circumoral ring of larvaceans.[14]
- dorsal strand
- Posterior extension of the neural gland.[15]
- dorsal tubercle
- Ciliated funnel connecting the neural gland to the branchial basket. Its shape varies between species, from a simple U-shaped funnel to a longer slit-like or more elaborate structure.[16]
- endostyle
- Mucus-secreting organ located inside the branchial basket.[5] The mucus net it produces lines the inner branchial basket, and captures food particles.[7] It is believed to be homologous to the thyroid in vertebrates.[17]
- gonozooid
- Sexual generation in the life cycle of doliolids. They grow from a stalk connecting the phorozooid to its parent oozooid. After the phorozooid breaks off, the gonozooids keep growing attached to its ventral peduncle, until themselves breaking off and becoming free-living. Gonozooids are hermaphroditic and reproduce by internal fertilization.[11]
- house
- A structure secreted by larvaceans, composed of oikosins and cellulose. It comprises several chambers, and fully surrounds the body in some species.[18] Houses are used for filter-feeding and to provide buoyancy, and are repeatedly discarded and replaced after becoming clogged.[19]
- hyaline cap
- Glue reservoir positioned at the anterior tip of the papillae, secreting adhesive material upon contact with the substrate.[20]
- mantle
-
Epidermal epithelium layer located below the tunic.[21]
- neural gland
- Gland connected to the brain, together forming the neural complex. Its exact function is unclear.[15] It is believed to be homologous to the anterior pituitary in vertebrates.[16]
- nurse
- Mature stage of the oozooid in doliolids, capable of producing phorozooids and trophozooids.[12]
- ocellus
- Light-sensing organ inside the sensory vesicle. It is multicellular, containing a cup-shaped pigment cell, three lens cells, and various kinds of photoreceptor cells.[22]
- oikoplast
-
Oikosin-producing layer of epithelium surrounding the trunk of larvaceans. The secreted oikosins form a pre-house above the epithelium before being inflated into the house. It is divided in multiple cellular fields, varying in cell morphology and involved in the formation of different parts of the house.[18]
- oikosin
- Glycoproteins constituting the larvacean house, secreted from the oikoplast.[18][19]
- oozooid
- Asexual generation in the life cycle of salps and doliolids. In both groups, oozooids reproduce by budding from a ventral stolon.[6]
- oral siphon
-
Opening through which water enters the branchial basket in ascidians.[5]
- otolith
-
Gravity-sensing organ inside the sensory vesicle.[23] It usually consists of a single large cell, with a foot extending to the ventral wall of the sensory vesicle and a round body containing a melanin granule.[22]
- papillae
-
Adhesive protrusions helping ascidian larvae to attach to their substrate. They are located at the anterior end of the larva, below the tunic.[24] In most species, three papillae are present. They are made of three types of cells: collocytes secreting an adhesive material, ciliated neurons playing a role in triggering metamorphosis, and axial columnar cells possessing sensory properties.[25] The papillae end in a hyaline cap.[4] They are usually conical in solitary tunicates, and eversible in colonial tunicates.[24] The set of papillae is also referred to as the adhesive organ.[3]
- peripharyngeal band
- phorozooid
- Asexual generation in the life cycle of doliolids. Phorozooids develop from buds produced by the oozooid having migrated to the paired central rows of the cadophore. They detach from the oozooid while carrying developing gonozooids on their ventral peduncles, before later releasing them.[11]
- sensory vesicle
- Main neural concentration in ascidian larvae. It contains the ocellus and otolith, while its posterior part is involved in brain activity.[26]
- siphon
- stigmata
-
Orifices in the branchial basket. They are lined with bands of cilia that help filter water. In ascidians, they can vary in shape, with some species having elongated or spiral stigmata.[7]
- stolon
- Structure connecting zooids to each other or to buds in a colony.[8]
- trophozooid
-
Asexually produced zooids in the life cycle of doliolids. They develop from buds produced by the oozooid having migrated to the lateral rows of the cadophore. Their role is to feed the colony, including the oozooid.[11]
- trunk ganglion
-
Enlarged region of the neural tube posterior to the sensory cavity, separated from it by a narrow neck region.[29]
- tunic
- Extracellular layer covering the epidermis in ascidians and thaliaceans. Fibers of biogenic cellulose, also called tunicin, make up the tunic's skeleton.[30] Free-living cells are present within the tunic, and play a role in its synthes and healing.[31]
- zooid
- Individual member of a tunicate colony.[8]
A
B
C

D
E
G

H
M
N
O

P

S
T
Z
References
- ^ a b Wanninger 2015, p. 136.
- ^ Wanninger 2015, p. 139.
- ^ a b Katz 1983, p. 10.
- ^ a b Zeng et al. 2019a, p. 184.
- ^ a b c Holland 2016, p. 147.
- ^ a b Deibel & Lowen 2012, pp. 359–360.
- ^ a b c Petersen & Svane 2002, p. 397.
- ^ a b c Brown & Swalla 2012, p. 152.
- ^ Zaniolo et al. 1998, p. 12.
- ^ Nakauchi 1982, p. 753–754.
- ^ a b c d Deibel & Lowen 2012, p. 360.
- ^ a b Greer et al. 2022, p. 193.
- ^ Caicci et al. 2013, p. 2756–2757.
- ^ Rigon et al. 2013, p. 1–2.
- ^ a b Braun & Stach 2019, p. 324.
- ^ a b Braun & Stach 2019, p. 339.
- ^ Takagi et al. 2022, p. 2.
- ^ a b c Hosp et al. 2012, p. 1.
- ^ a b Holland 2016, p. 148.
- ^ Zeng et al. 2019b, p. 2.
- ^ Di Bella, Carbone & De Leo 2005, p. 477–478.
- ^ a b Hudson 2016, p. 6.
- ^ Jiang et al. 2005, p. 435.
- ^ a b Pennati & Rothbächer 2015, p. 2.
- ^ Johnson et al. 2024, p. 1–2.
- ^ Hudson 2016, p. 6–7.
- ^ Petersen & Svane 2002, p. 401.
- ^ Holland 2016, p. 150.
- ^ Hudson 2016, p. 8.
- ^ Zhao & Li 2014, p. 3428–3429.
- ^ Di Bella, Carbone & De Leo 2005, p. 477.
Works cited
- Anselmi, Chiara; Fuller, Gwynna K.; Stolfi, Alberto; Groves, Andrew K.; Manni, Lucia (2024-03-14). "Sensory cells in tunicates: insights into mechanoreceptor evolution". Frontiers in Cell and Developmental Biology. 12. doi:10.3389/fcell.2024.1359207. ISSN 2296-634X. PMC 10973136. PMID 38550380.
- Braun, Katrin; Stach, Thomas (May 2019). "Morphology and evolution of the central nervous system in adult tunicates". Journal of Zoological Systematics and Evolutionary Research. 57 (2): 323–344. doi:10.1111/jzs.12246. ISSN 0947-5745.
- Brown, Federico D.; Swalla, Billie J. (September 2012). "Evolution and development of budding by stem cells: Ascidian coloniality as a case study". Developmental Biology. 369 (2): 151–162. doi:10.1016/j.ydbio.2012.05.038. PMID 22722095.
- Caicci, Federico; Gasparini, Fabio; Rigon, Francesca; Zaniolo, Giovanna; Burighel, Paolo; Manni, Lucia (2013-08-15). "The oral sensory structures of Thaliacea (Tunicata) and consideration of the evolution of hair cells in chordata". Journal of Comparative Neurology. 521 (12): 2756–2771. doi:10.1002/cne.23313. ISSN 0021-9967. PMID 23386364.
- Deibel, Don; Lowen, Ben (2012-05-01). "A review of the life cycles and life-history adaptations of pelagic tunicates to environmental conditions". ICES Journal of Marine Science. 69 (3): 358–369. doi:10.1093/icesjms/fsr159. ISSN 1095-9289.
- Di Bella, Maria Antonietta; Carbone, Maria Carmela; De Leo, Giacomo (2005-01-17). "Aspects of cell production in mantle tissue of Ciona intestinalis L. (Tunicata, Ascidiacea)". Micron. 36 (5): 477–481. doi:10.1016/j.micron.2005.01.007. hdl:10447/3224. PMID 15935306.
- Greer, Adam T.; Schmid, Moritz S.; Duffy, Patrick I.; Robinson, Kelly L.; Genung, Mark A.; Luo, Jessica Y.; Panaïotis, Thelma; Briseño-Avena, Christian; Frischer, Marc E.; Sponaugle, Su; Cowen, Robert K. (2022-11-26). "In situ imaging across ecosystems to resolve the fine-scale oceanographic drivers of a globally significant planktonic grazer". Limnology and Oceanography. 68 (1): 192–207. doi:10.1002/lno.12259. ISSN 0024-3590.
- Holland, Linda Z. (2016-02-22). "Tunicates". Current Biology. 26 (4): R146–R152. Bibcode:2016CBio...26.R146H. doi:10.1016/j.cub.2015.12.024. PMID 26906481.
- Hosp, Julia; Sagane, Yoshimasa; Danks, Gemma; Thompson, Eric M. (2012-07-05). Schubert, Michael (ed.). "The Evolving Proteome of a Complex Extracellular Matrix, the Oikopleura House". PLOS ONE. 7 (7): e40172. Bibcode:2012PLoSO...740172H. doi:10.1371/journal.pone.0040172. ISSN 1932-6203. PMC 3390340. PMID 22792236.
- Hudson, Clare (June 2016). "The central nervous system of ascidian larvae". WIREs Developmental Biology. 5 (5): 538–561. doi:10.1002/wdev.239. ISSN 1759-7684. PMID 27328318.
- Jiang, Di; Tresser, Jason W.; Horie, Takeo; Tsuda, Motoyuki; Smith, William C. (2005-02-01). "Pigmentation in the sensory organs of the ascidian larva is essential for normal behavior". Journal of Experimental Biology. 208 (3): 433–438. Bibcode:2005JExpB.208..433J. doi:10.1242/jeb.01420. ISSN 1477-9145. PMID 15671331.
- Johnson, Christopher J.; Razy-Krajka, Florian; Zeng, Fan; Piekarz, Katarzyna M.; Biliya, Shweta; Rothbächer, Ute; Stolfi, Alberto (2024-03-13). Fernandez-Valverde, Selene L (ed.). "Specification of distinct cell types in a sensory-adhesive organ important for metamorphosis in tunicate larvae". PLOS Biology. 22 (3): e3002555. doi:10.1371/journal.pbio.3002555. ISSN 1545-7885. PMC 10962819. PMID 38478577.
- Katz, Michael J. (1983). "Comparative Anatomy of the Tunicate Tadpole, Ciona intestinalis". Biological Bulletin. 164 (1): 1–27. doi:10.2307/1541186. ISSN 0006-3185. JSTOR 1541186.
- Nakauchi, Mitsuaki (November 1982). "Asexual Development of Ascidians: Its Biological Significance, Diversity, and Morphogenesis". American Zoologist. 22 (4): 753–763. doi:10.1093/icb/22.4.753. ISSN 0003-1569.
- Pennati, Roberta; Rothbächer, Ute (2015-02-06). "Bioadhesion in ascidians: a developmental and functional genomics perspective". Interface Focus. 5 (1): 20140061. doi:10.1098/rsfs.2014.0061. ISSN 2042-8898. PMC 4275875. PMID 25657840.
- Petersen, J.; Svane, I. (2002-02-01). "Filtration rate in seven Scandinavian ascidians: implications of the morphology of the gill sac". Marine Biology. 140 (2): 397–402. doi:10.1007/s002270100706. ISSN 0025-3162.
- Rigon, Francesca; Stach, Thomas; Caicci, Federico; Gasparini, Fabio; Burighel, Paolo; Manni, Lucia (2013-06-04). "Evolutionary diversification of secondary mechanoreceptor cells in tunicata". BMC Evolutionary Biology. 13 (1): 112. Bibcode:2013BMCEE..13..112R. doi:10.1186/1471-2148-13-112. ISSN 1471-2148. PMC 3682859. PMID 23734698.
- Takagi, Wataru; Sugahara, Fumiaki; Higuchi, Shinnosuke; Kusakabe, Rie; Pascual-Anaya, Juan; Sato, Iori; Oisi, Yasuhiro; Ogawa, Nobuhiro; Miyanishi, Hiroshi; Adachi, Noritaka; Hyodo, Susumu; Kuratani, Shigeru (2022-04-01). "Thyroid and endostyle development in cyclostomes provides new insights into the evolutionary history of vertebrates". BMC Biology. 20 (1): 76. doi:10.1186/s12915-022-01282-7. ISSN 1741-7007. PMC 8973611. PMID 35361194.
- Wanninger, Andreas (2015). Evolutionary Developmental Biology of Invertebrates 6: Deuterostomia (1st ed. 2015 ed.). Vienna: Springer. doi:10.1007/978-3-7091-1856-6_4. ISBN 978-3-7091-1856-6.
- Zaniolo, G.; Manni, L.; Brunetti, R.; Burighel, P. (January 1998). "Brood pouch differentiation in Botrylloides violaceus, a viviparous ascidian (Tunicata)". Invertebrate Reproduction & Development. 33 (1): 11–23. Bibcode:1998InvRD..33...11Z. doi:10.1080/07924259.1998.9652338. hdl:11577/2470037. ISSN 0792-4259.
- Zeng, Fan; Wunderer, Julia; Salvenmoser, Willi; Hess, Michael W.; Ladurner, Peter; Rothbächer, Ute (2019-04-15). "Papillae revisited and the nature of the adhesive secreting collocytes". Developmental Biology. Current Directions in Tunicate Development. 448 (2): 183–198. doi:10.1016/j.ydbio.2018.11.012. ISSN 0012-1606. PMID 30471266.
- Zeng, Fan; Wunderer, Julia; Salvenmoser, Willi; Ederth, Thomas; Rothbächer, Ute (2019-10-28). "Identifying adhesive components in a model tunicate". Philosophical Transactions of the Royal Society B: Biological Sciences. 374 (1784): 20190197. doi:10.1098/rstb.2019.0197. ISSN 0962-8436. PMC 6745482. PMID 31495315.
- Zhao, Yadong; Li, Jiebing (2014-07-05). "Excellent chemical and material cellulose from tunicates: diversity in cellulose production yield and chemical and morphological structures from different tunicate species". Cellulose. 21 (5): 3427–3441. doi:10.1007/s10570-014-0348-6. ISSN 0969-0239.
