Fludarabine
 | |
| Clinical data | |
|---|---|
| Trade names | Fludara, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692003 |
| Routes of administration | Intravenous, by mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 55% |
| Protein binding | 19 to 29% |
| Elimination half-life | 20 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.123.703 |
| Chemical and physical data | |
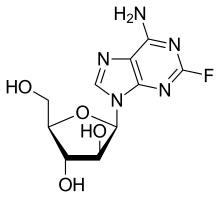
| Formula | C10H12FN5O4 |
| Molar mass | 285.235 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fludarabine is a purine analogue and antineoplastic agent. It is generally used as its 5-O-phosphorylated form known as fludarabine phosphate, sold under the brand name Fludara among others. It is a chemotherapy medication used in the treatment of leukemia and lymphoma.[3] These include chronic lymphocytic leukemia, non-Hodgkin's lymphoma, acute myeloid leukemia, and acute lymphocytic leukemia.[3] It is given by injection into a vein or by mouth.[3]
Common side effects include nausea, diarrhea, fever, rash, shortness of breath, numbness, vision changes, and feeling tired.[3] Severe side effects include brain dysfunction, low blood cell counts, and lung inflammation.[3] Use in pregnancy will likely result in harm to the fetus.[3] Fludarabine is in the purine analog family of medications and works by interfering with the duplication of DNA.[3][4]
Fludarabine was approved for medical use in the United States in 1991.[3] It is on the World Health Organization's List of Essential Medicines.[5]
Medical uses
Fludarabine is highly effective in the treatment of chronic lymphocytic leukemia, producing higher response rates than alkylating agents such as chlorambucil alone.[6] Fludarabine is used in various combinations with cyclophosphamide, mitoxantrone, dexamethasone and rituximab in the treatment of indolent non-Hodgkin's lymphomas. As part of the FLAG or FLAMSA regimen, fludarabine is used together with cytarabine and granulocyte colony-stimulating factor in the treatment of acute myeloid leukaemia. Because of its immunosuppressive effects, fludarabine is also used in some conditioning regimens prior to allogeneic stem cell transplant.
Side effects
Fludarabine is associated with profound lymphopenia, and as a consequence, increases the risk of opportunistic infections. People who have been treated with fludarabine will usually be asked to take co-trimoxazole or to use monthly nebulised pentamidine to prevent Pneumocystis jiroveci pneumonia. The profound lymphopenia caused by fludarabine renders patients susceptible to transfusion-associated graft versus host disease, an oftentimes fatal complication of blood transfusion. For this reason, all patients who have ever received fludarabine should only be given irradiated blood components.
Fludarabine causes anemia, thrombocytopenia and neutropenia, requiring regular blood count monitoring. Some patients require blood and platelet transfusion, or G-CSF injections to boost neutrophil counts.
Fludarabine is associated with the development of severe autoimmune hemolytic anemia in a proportion of patients.[7]
Difficulties are often encountered when harvesting peripheral blood stem cells from patients previously treated with fludarabine.[8]
Pharmacology
Fludarabine is a purine analog, and can be given both orally and intravenously. Fludarabine inhibits DNA synthesis by interfering with ribonucleotide reductase and DNA polymerase. It is active against both dividing and resting cells. Being phosphorylated, fludarabine is ionized at physiologic pH and is effectually trapped in blood. This provides some level of specificity for blood cells, both cancerous and healthy.
History
Fludarabine was produced by John Montgomery and Kathleen Hewson of the Southern Research Institute in 1968.[9]
Names
Fludarabine is generally administered as its 5-O-phosphorylated form known as fludarabine phosphate, which is rapidly dephosphorylated to fludarabine in the plasma.
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ "Product monograph brand safety updates". Health Canada. 7 July 2016. Retrieved 3 April 2024.
- ^ a b c d e f g h "Fludarabine Phosphate". The American Society of Health-System Pharmacists. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
- ^ Helms RA, Quan DJ (2006). Textbook of Therapeutics: Drug and Disease Management. Lippincott Williams & Wilkins. p. 2309. ISBN 9780781757348. Archived from the original on 2016-12-20.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Rai KR, Peterson BL, Appelbaum FR, Kolitz J, Elias L, Shepherd L, et al. (December 2000). "Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia". The New England Journal of Medicine. 343 (24): 1750–1757. doi:10.1056/NEJM200012143432402. PMID 11114313.
- ^ Gonzalez H, Leblond V, Azar N, Sutton L, Gabarre J, Binet JL, et al. (June 1998). "Severe autoimmune hemolytic anemia in eight patients treated with fludarabine". Hematology and Cell Therapy. 40 (3): 113–118. PMID 9698219.
- ^ Tournilhac O, Cazin B, Leprètre S, Diviné M, Maloum K, Delmer A, et al. (January 2004). "Impact of frontline fludarabine and cyclophosphamide combined treatment on peripheral blood stem cell mobilization in B-cell chronic lymphocytic leukemia". Blood. 103 (1): 363–365. doi:10.1182/blood-2003-05-1449. PMID 12969985.
- ^ Sneader W (2005). Drug discovery: a history. New York: Wiley. p. 258. ISBN 0-471-89979-8.
