FGI-103
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
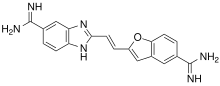
| Formula | C19H16N6O |
| Molar mass | 344.378 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
FGI-103 is an antiviral drug developed as a potential treatment for the filoviruses Ebola virus and Marburg virus. In tests on mice FGI-103 was effective against both Ebola and Marburg viruses when administered up to 48 hours after infection. The mechanism of action of FGI-103 has however not yet been established, as it was found not to be acting by any of the known mechanisms used by similar antiviral drugs.[1][2][3]
See also
References
- ^ Warren TK, Warfield KL, Wells J, Enterlein S, Smith M, Ruthel G, et al. (May 2010). "Antiviral activity of a small-molecule inhibitor of filovirus infection". Antimicrobial Agents and Chemotherapy. 54 (5): 2152–9. doi:10.1128/AAC.01315-09. PMC 2863630. PMID 20211898.
- ^ Bradfute SB, Warfield KL, Bray M (September 2012). "Mouse models for filovirus infections". Viruses. 4 (9): 1477–508. doi:10.3390/v4091477. PMC 3499815. PMID 23170168.
- ^ De Clercq E (January 2015). "Ebola virus (EBOV) infection: Therapeutic strategies". Biochemical Pharmacology. 93 (1): 1–10. doi:10.1016/j.bcp.2014.11.008. PMC 7110990. PMID 25481298.
