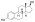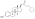Estradiol glucuronide

| |
| Names | |
|---|---|
| IUPAC name 3-Hydroxyestra-1,3,5(10)-trien-17β-yl β-D-glucopyranosiduronic acid | |
| Systematic IUPAC name (2S,3S,4S,5R,6R)-3,4,5-Trihydroxy-6-{[(1S,3aS,3bR,9bS,11aS)-7-hydroxy-11a-methyl-2,3,3a,3b,4,5,9b,10,11,11a-decahydro-1H-cyclopenta[a]phenanthren-1-yl]oxy}oxane-2-carboxylic acid | |
| Other names E217βG; 17β-Estradiol 17β-D-glucuronide; Estra-1,3,5(10)-triene-3,17β-diol 17β-D-glucuronoside | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C24H32O8 | |
| Molar mass | 448.512 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Estradiol glucuronide, or estradiol 17β-D-glucuronide, is a conjugated metabolite of estradiol.[1] It is formed from estradiol in the liver by UDP-glucuronyltransferase via attachment of glucuronic acid and is eventually excreted in the urine by the kidneys.[1] It has much higher water solubility than does estradiol.[1] Glucuronides are the most abundant estrogen conjugates.[1]
When exogenous estradiol is administered orally, it is subject to extensive first-pass metabolism (95%) in the intestines and liver.[2][3] A single administered dose of estradiol is absorbed 15% as estrone, 25% as estrone sulfate, 25% as estradiol glucuronide, and 25% as estrone glucuronide.[2] Formation of estrogen glucuronide conjugates is particularly important with oral estradiol as the percentage of estrogen glucuronide conjugates in circulation is much higher with oral ingestion than with parenteral estradiol.[2] Estradiol glucuronide can be converted back into estradiol, and a large circulating pool of estrogen glucuronide and sulfate conjugates serves as a long-lasting reservoir of estradiol that effectively extends its elimination half-life of oral estradiol.[2] In demonstration of the importance of first-pass metabolism and the estrogen conjugate reservoir in the pharmacokinetics of estradiol,[2] the elimination half-life of oral estradiol is 13 to 20 hours[4] whereas with intravenous injection its elimination half-life is only about 1 to 2 hours.[5]
Approximately 7% of estradiol is excreted in the urine as estradiol glucuronide.[6]
Estradiol glucuronide is transported into prostate gland, testis, and breast cells by OATP1A2, OATP1B1, OATP1B3, OATP1C1, and OATP3A1.[7] The ABC transporters MRP2, MRP3, MRP4, and BCRP, as well as several other transporters, have been found to transport estradiol glucuronide out of cells.[7][8]
The circulating concentrations of estrogen glucuronides are generally more than 10-fold lower than those of estrone sulfate, the most abundant estrogen conjugate in the circulation.[8]
Estradiol glucuronide has been identified as an agonist of the G protein-coupled estrogen receptor (GPER), a membrane estrogen receptor.[9] This may be involved in estradiol glucuronide-induced cholestasis.[9]
Estrogen glucuronides can be deglucuronidated into the corresponding free estrogens by β-glucuronidase in tissues that express this enzyme, such as the mammary gland.[10] As a result, estrogen glucuronides have estrogenic activity via conversion into estrogens.[10]
Estradiol glucuronide shows about 300-fold lower potency in activating the estrogen receptors relative to estradiol in vitro.[11]
The positional isomer of estradiol glucuronide, estradiol 3-glucuronide, also occurs as a major endogenous metabolite of estradiol, circulating at two-thirds of the levels of estrone sulfate when it reaches its maximal concentrations just before ovulation and during the peak in estradiol levels that occurs at this time.[12]
| Estrogen | Other names | RBA (%)a | REP (%)b | |||
|---|---|---|---|---|---|---|
| ER | ERα | ERβ | ||||
| Estradiol | E2 | 100 | 100 | 100 | ||
| Estradiol 3-sulfate | E2S; E2-3S | ? | 0.02 | 0.04 | ||
| Estradiol 3-glucuronide | E2-3G | ? | 0.02 | 0.09 | ||
| Estradiol 17β-glucuronide | E2-17G | ? | 0.002 | 0.0002 | ||
| Estradiol benzoate | EB; Estradiol 3-benzoate | 10 | 1.1 | 0.52 | ||
| Estradiol 17β-acetate | E2-17A | 31–45 | 24 | ? | ||
| Estradiol diacetate | EDA; Estradiol 3,17β-diacetate | ? | 0.79 | ? | ||
| Estradiol propionate | EP; Estradiol 17β-propionate | 19–26 | 2.6 | ? | ||
| Estradiol valerate | EV; Estradiol 17β-valerate | 2–11 | 0.04–21 | ? | ||
| Estradiol cypionate | EC; Estradiol 17β-cypionate | ?c | 4.0 | ? | ||
| Estradiol palmitate | Estradiol 17β-palmitate | 0 | ? | ? | ||
| Estradiol stearate | Estradiol 17β-stearate | 0 | ? | ? | ||
| Estrone | E1; 17-Ketoestradiol | 11 | 5.3–38 | 14 | ||
| Estrone sulfate | E1S; Estrone 3-sulfate | 2 | 0.004 | 0.002 | ||
| Estrone glucuronide | E1G; Estrone 3-glucuronide | ? | <0.001 | 0.0006 | ||
| Ethinylestradiol | EE; 17α-Ethynylestradiol | 100 | 17–150 | 129 | ||
| Mestranol | EE 3-methyl ether | 1 | 1.3–8.2 | 0.16 | ||
| Quinestrol | EE 3-cyclopentyl ether | ? | 0.37 | ? | ||
| Footnotes: a = Relative binding affinities (RBAs) were determined via in-vitro displacement of labeled estradiol from estrogen receptors (ERs) generally of rodent uterine cytosol. Estrogen esters are variably hydrolyzed into estrogens in these systems (shorter ester chain length -> greater rate of hydrolysis) and the ER RBAs of the esters decrease strongly when hydrolysis is prevented. b = Relative estrogenic potencies (REPs) were calculated from half-maximal effective concentrations (EC50) that were determined via in-vitro β‐galactosidase (β-gal) and green fluorescent protein (GFP) production assays in yeast expressing human ERα and human ERβ. Both mammalian cells and yeast have the capacity to hydrolyze estrogen esters. c = The affinities of estradiol cypionate for the ERs are similar to those of estradiol valerate and estradiol benzoate (figure). Sources: See template page. | ||||||
| Estrogen | Structure | Ester(s) | Relative mol. weight |
Relative E2 contentb |
log Pc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position(s) | Moiet(ies) | Type | Lengtha | ||||||
| Estradiol | – | – | – | – | 1.00 | 1.00 | 4.0 | ||
| Estradiol acetate | C3 | Ethanoic acid | Straight-chain fatty acid | 2 | 1.15 | 0.87 | 4.2 | ||
| Estradiol benzoate | C3 | Benzoic acid | Aromatic fatty acid | – (~4–5) | 1.38 | 0.72 | 4.7 | ||
| Estradiol dipropionate | C3, C17β | Propanoic acid (×2) | Straight-chain fatty acid | 3 (×2) | 1.41 | 0.71 | 4.9 | ||
| Estradiol valerate | C17β | Pentanoic acid | Straight-chain fatty acid | 5 | 1.31 | 0.76 | 5.6–6.3 | ||
| Estradiol benzoate butyrate | C3, C17β | Benzoic acid, butyric acid | Mixed fatty acid | – (~6, 2) | 1.64 | 0.61 | 6.3 | ||
| Estradiol cypionate | C17β | Cyclopentylpropanoic acid | Cyclic fatty acid | – (~6) | 1.46 | 0.69 | 6.9 | ||
| Estradiol enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | 6.7–7.3 | ||
| Estradiol dienanthate | C3, C17β | Heptanoic acid (×2) | Straight-chain fatty acid | 7 (×2) | 1.82 | 0.55 | 8.1–10.4 | ||
| Estradiol undecylate | C17β | Undecanoic acid | Straight-chain fatty acid | 11 | 1.62 | 0.62 | 9.2–9.8 | ||
| Estradiol stearate | C17β | Octadecanoic acid | Straight-chain fatty acid | 18 | 1.98 | 0.51 | 12.2–12.4 | ||
| Estradiol distearate | C3, C17β | Octadecanoic acid (×2) | Straight-chain fatty acid | 18 (×2) | 2.96 | 0.34 | 20.2 | ||
| Estradiol sulfate | C3 | Sulfuric acid | Water-soluble conjugate | – | 1.29 | 0.77 | 0.3–3.8 | ||
| Estradiol glucuronide | C17β | Glucuronic acid | Water-soluble conjugate | – | 1.65 | 0.61 | 2.1–2.7 | ||
| Estramustine phosphated | C3, C17β | Normustine, phosphoric acid | Water-soluble conjugate | – | 1.91 | 0.52 | 2.9–5.0 | ||
| Polyestradiol phosphatee | C3–C17β | Phosphoric acid | Water-soluble conjugate | – | 1.23f | 0.81f | 2.9g | ||
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic or cyclic fatty acids. b = Relative estradiol content by weight (i.e., relative estrogenic exposure). c = Experimental or predicted octanol/water partition coefficient (i.e., lipophilicity/hydrophobicity). Retrieved from PubChem, ChemSpider, and DrugBank. d = Also known as estradiol normustine phosphate. e = Polymer of estradiol phosphate (~13 repeat units). f = Relative molecular weight or estradiol content per repeat unit. g = log P of repeat unit (i.e., estradiol phosphate). Sources: See individual articles. | |||||||||
See also
- Catechol estrogen
- Estradiol sulfate
- Estriol glucuronide
- Estriol sulfate
- Estrogen conjugate
- Lipoidal estradiol
- List of estrogen esters § Estradiol esters
References
- ^ a b c d "Human Metabolome Database: Showing metabocard for 17-beta-Estradiol glucuronide (HMDB0010317)".
- ^ a b c d e Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 268–. ISBN 978-3-642-60107-1.
- ^ M. Notelovitz; P.A. van Keep (6 December 2012). The Climacteric in Perspective: Proceedings of the Fourth International Congress on the Menopause, held at Lake Buena Vista, Florida, October 28–November 2, 1984. Springer Science & Business Media. pp. 406–. ISBN 978-94-009-4145-8.
- ^ Stanczyk, Frank Z.; Archer, David F.; Bhavnani, Bhagu R. (2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–727. doi:10.1016/j.contraception.2012.12.011. ISSN 0010-7824. PMID 23375353.
- ^ Düsterberg B, Nishino Y (1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas. 4 (4): 315–24. doi:10.1016/0378-5122(82)90064-0. PMID 7169965.
- ^ Kelly Smith; Daniel M. Riche; Nickole Henyan (15 April 2010). Clinical Drug Data, 11th Edition. McGraw Hill Professional. ISBN 978-0-07-162686-6.
- ^ a b Mueller JW, Gilligan LC, Idkowiak J, Arlt W, Foster PA (October 2015). "The Regulation of Steroid Action by Sulfation and Desulfation". Endocr. Rev. 36 (5): 526–63. doi:10.1210/er.2015-1036. PMC 4591525. PMID 26213785.
- ^ a b Järvinen E, Deng F, Kidron H, Finel M (April 2018). "Efflux transport of estrogen glucuronides by human MRP2, MRP3, MRP4 and BCRP". J. Steroid Biochem. Mol. Biol. 178: 99–107. doi:10.1016/j.jsbmb.2017.11.007. hdl:10138/321530. PMID 29175180. S2CID 3678002.
- ^ a b Zucchetti AE, Barosso IR, Boaglio AC, Basiglio CL, Miszczuk G, Larocca MC, Ruiz ML, Davio CA, Roma MG, Crocenzi FA, Pozzi EJ (March 2014). "G-protein-coupled receptor 30/adenylyl cyclase/protein kinase A pathway is involved in estradiol 17β-D-glucuronide-induced cholestasis". Hepatology. 59 (3): 1016–29. doi:10.1002/hep.26752. hdl:2133/10484. PMID 24115158. S2CID 20568614.
- ^ a b Zhu BT, Conney AH (January 1998). "Functional role of estrogen metabolism in target cells: review and perspectives". Carcinogenesis. 19 (1): 1–27. doi:10.1093/carcin/19.1.1. PMID 9472688.
- ^ Coldham NG, Dave M, Sivapathasundaram S, McDonnell DP, Connor C, Sauer MJ (July 1997). "Evaluation of a recombinant yeast cell estrogen screening assay". Environ. Health Perspect. 105 (7): 734–42. doi:10.1289/ehp.97105734. PMC 1470103. PMID 9294720.
- ^ F. A. Kincl; J. R. Pasqualini (22 October 2013). Hormones and the Fetus: Volume 1: Production, Concentration and Metabolism During Pregnancy. Elsevier Science. pp. 39–. ISBN 978-1-4832-8538-2.