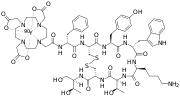
DOTA (chelator)

| |
| Names | |
|---|---|
| Preferred IUPAC name 2,2′,2′′,2′′′-(1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetic acid | |
| Other names DotA; Tetraxetan | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.113.833 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H28N4O8 | |
| Molar mass | 404.420 g·mol−1 |
| Appearance | White crystalline solid |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P305+P351+P338 | |
| Related compounds | |
Related compounds |
Cyclen, EDTA |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
DOTA (also known as tetraxetan) is an organic compound with the formula (CH2CH2NCH2CO2H)4. The molecule consists of a central 12-membered tetraaza (i.e., containing four nitrogen atoms) ring. DOTA is used as a complexing agent, especially for lanthanide ions. Its complexes have medical applications as contrast agents and cancer treatments.
Terminology
The acronym DOTA (for dodecane tetraacetic acid) is shorthand for both the tetracarboxylic acid and its various conjugate bases. In the area of coordination chemistry, the tetraacid is called H4DOTA and its fully deprotonated derivative is DOTA4−.[1] Many related ligands are referred to using the DOTA acronym, although these derivatives are generally not tetracarboxylic acids or the conjugate bases.
Structure
DOTA is derived from the macrocycle known as cyclen. The four secondary amine groups are modified by replacement of the N-H centers with N-CH2CO2H groups. The resulting aminopolycarboxylic acid, upon ionization of the carboxylic acid groups, is a high affinity chelating agent for di- and trivalent cations. The tetracarboxylic acid was first reported in 1976.[2] At the time of its discovery DOTA exhibited the largest known formation constant for the complexation (chelating) of Ca2+ and Gd3+ ions. Modified versions of DOTA were first reported in 1988 and this area has proliferated since.[3][4]
As a polydentate ligand, DOTA envelops metal cations, but the denticity of the ligand depends on the geometric tendencies of the metal cation. The main applications involve the lanthanides and in such complexes DOTA functions as an octadentate ligand, binding the metal through four amine and four carboxylate groups. Most such complexes feature an additional water ligand, giving an overall coordination number of nine.[1]
For most transition metals, DOTA functions as a hexadentate ligand, binding through the four nitrogen and two carboxylate centres. The complexes have octahedral coordination geometry, with two pendent carboxylate groups. In the case of [Fe(DOTA)]−, the ligand is heptadentate.[1]
Uses
Cancer treatment and diagnosis
DOTA can be conjugated to monoclonal antibodies by attachment of one of the four carboxyl groups as an amide. The remaining three carboxylate anions are available for binding to the yttrium ion. The modified antibody accumulates in the tumour cells, concentrating the effects of the radioactivity of 90Y. Drugs containing this module receive an International Nonproprietary Name ending in tetraxetan:[5]
DOTA can also be linked to molecules that have affinity for various structures. The resulting compounds are used with a number of radioisotopes in cancer therapy and diagnosis (for example in positron emission tomography).
- Affinity for somatostatin receptors, which are found on neuroendocrine tumours:[6]
- Affinity for the proteins streptavidin and avidin, which can be targeted at tumours by aid of monoclonal antibodies:[7]
- DOTA linked to the monoclonal antibody tacatuzumab and chelating yttrium-90
- DOTATOC chelating yttrium-90
Contrast agent
The complex of Gd3+ and DOTA is used as a gadolinium-based MRI contrast agent under the name gadoteric acid.[8]
Synthesis
DOTA was first synthesized in 1976 from cyclen and bromoacetic acid.[2] This method is simple and still in use.[9]
References
- ^ a b c Viola-Villegas, Nerissa; Doyle, Robert P (2009). "The coordination chemistry of 1,4,7,10-tetraazacyclododecane-N,N′,N",N′"-tetraacetic acid (H4DOTA): Structural overview and analyses on structure–stability relationships". Coordination Chemistry Reviews. 253 (13–14): 1906. doi:10.1016/j.ccr.2009.03.013.
- ^ a b Stetter, Hermann; Wolfram Frank (1976). "Complex Formation with Tetraazacycloalkane-N,N',N'',N''';-tetraacetic Acids as a Function of Ring Size". Angewandte Chemie International Edition in English. 15 (11): 686. doi:10.1002/anie.197606861.
- ^ Moi, Min K.; Claude F. Meares; Sally J. DeNardo (1988). "The peptide way to macrocyclic bifunctional chelating agents: synthesis of 2-(p-nitrobenzyl)-1,4,7,10-tetraazacyclododecane-N,N',N'',N'''-tetraacetic acid and study of its yttrium(III) complex". Journal of the American Chemical Society. 110 (18): 6266–6267. doi:10.1021/ja00226a063. PMID 22148823.
- ^ Volkert, Wynn A.; Timothy J. Hoffman (1999). "Therapeutic Radiopharmaceuticals". Chemical Reviews. 99 (9): 2269–2292. doi:10.1021/cr9804386. PMID 11749482.
- ^ "Statement On A Nonproprietary Name Adopted By The USAN Council: Yttrium Y90 clivatuzumab tetraxetan" (PDF). American Medical Association.
- ^ Breeman, W. A. P.; De Blois, E.; Sze Chan, H.; Konijnenberg, M.; Kwekkeboom, D. J.; Krenning, E. P. (2011). "68Ga-labeled DOTA-Peptides and 68Ga-labeled Radiopharmaceuticals for Positron Emission Tomography: Current Status of Research, Clinical Applications, and Future Perspectives". Seminars in Nuclear Medicine. 41 (4): 314–321. doi:10.1053/j.semnuclmed.2011.02.001. PMID 21624565.
- ^ Domingo, R. J.; Reilly, R. M. (2000). "Pre-targeted radioimmunotherapy of human colon cancer xenografts in athymic mice using streptavidin-CC49 monoclonal antibody and 90Y-DOTA-biotin". Nuclear Medicine Communications. 21 (1): 89–96. doi:10.1097/00006231-200001000-00015. PMID 10717908.
- ^ A Clinical Study of Gadoteric Acid in Non-Coronary Magnetic Resonance (MR) Angiography, 25 November 2008
- ^ Knör, S; Modlinger, A; Poethko, T; Schottelius, M; Wester, HJ; Kessler, H (2007). "Synthesis of novel 1,4,7,10-tetraazacyclodecane-1,4,7,10-tetraacetic acid (DOTA) derivatives for chemoselective attachment to unprotected polyfunctionalized compounds". Chemistry: A European Journal. 13 (21): 6082–90. doi:10.1002/chem.200700231. PMID 17503419.