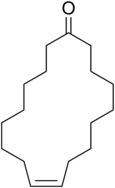
Civetone
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name (9Z)-Cycloheptadec-9-en-1-one | |||
| Other names cis-Civetone; 9-Cycloheptadecen-1-one; Cycloheptadeca-9-en-1-one; (Z)-9-Cyclohepta-decen-1-one | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.008.013 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C17H30O | |||
| Molar mass | 250.426 g·mol−1 | ||
| Appearance | Crystalline solid | ||
| Density | 0.917 at 33 °C | ||
| Melting point | 31 to 32 °C (88 to 90 °F; 304 to 305 K) | ||
| Boiling point | 342 °C (648 °F; 615 K) | ||
| Solubility in oils | soluble | ||
| Solubility in ethanol | soluble | ||
| Solubility in water | slightly soluble | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Civetone is a macrocyclic ketone and the main odorous constituent of civet oil.[1] It is a pheromone sourced from the African civet. It has a strong musky odor that becomes pleasant at extreme dilutions.[2] Civetone is closely related to muscone, the principal odoriferous compound found in musk; the structure of both compounds was elucidated by Leopold Ružička.[3] Today, civetone can be synthesized from precursor chemicals found in palm oil.[4]
Uses
Civetone is a synthetic musk used as a perfume fixative and flavor.
In order to attract jaguars to camera traps, field biologists have used the Calvin Klein-brand male cologne Obsession. It is believed that the civetone in the cologne resembles a territorial marking.[5]
See also
- 5-Cyclohexadecenone, a related musk chemical
References
- ^ The Merck Index, 15th Ed. (2013), p. 418, Monograph 2334, O'Neil: The Royal Society of Chemistry. Available online at: http://www.rsc.org/Merck-Index/monograph/mono1500002334
- ^ Bedoukian, Paul Z. "Perfumery and Flavoring Synthetics", 2nd ed., p. 248, Elsevier, New York, 1967.
- ^ Sell, Charles S. (1999). "Ingredients for the Modern Perfumery Industry". In Pybus, David H.; Sell, Charles S. (eds.). The Chemistry of Fragrances (1st ed.). Royal Society of Chemistry Publishing. pp. 51–124. ISBN 9780854045280.
- ^ Yuen-May Choo, Kay-Eng Ooi and Ing-Hong Ooi (August 1994). "Synthesis of civetone from palm oil products". Journal of the American Oil Chemists' Society. 71 (8). Springer Berlin / Heidelberg: 911–913. doi:10.1007/bf02540473. ISSN 0003-021X. S2CID 85189919.
- ^ "You'll Never Guess How Biologists Lure Jaguars To Camera Traps". Scientific American Blog Network.