Circulene
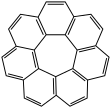
A circulene is a macrocyclic arene in which a central polygon is surrounded and fused by benzenoids.[1] Nomenclature within this class of molecules is based on the number of benzene rings surrounding the core, which is equivalent to the size of the central polygon. Examples which have been synthesized include [5]circulene (corannulene), [6]circulene (coronene), [7]circulene,[2][3][4][5] and [12]circulene (kekulene) These compounds belong to a larger class of geodesic polyarenes. Whereas [5]circulene is bowl-shaped and [6]circulene is planar, [7]circulene has a unique saddle-shaped structure (compare to cones and partial cones in calixarenes). The helicenes are a conceptually related class of structures in which the array of benzene rings form an open helix rather than a closed ring.
Quadrannulene ([4]circulene)
The simple [4]circulene compound itself has not been synthesized, but a derivative, tetrabenzo[4]circulene, also called quadrannulene, has.[6]
[8]Circulenes
The isolation of the [8]circulene derivative 2,5,6,9,10,13,14-octamethyl-3,4,7,8,11,12,15,16-octa(4-tolyl)[8]circulene has been reported.[7] Tetrabenzo[8]circulene (TB8C), a functionally stable form of the parent molecule [8]circulene has also been reported.[8][9][10]
Heterocyclic circulenes

A heterocyclic circulene is one in which the fused rings around the periphery are not simple hydrocarbons, but instead contain at least one other element. Sulflower is a stable heterocyclic octacirculene based on thiophene.
Gallery
- Corannulene ([5]circulene)
- Coronene ([6]circulene)
- [7]circulene
- Kekulene ([12]circulene)
References
- ^ Dopper, J.H.; Wynberg, Hans (1972). "Heterocirculenes a new class of polycyclic aromatic hydrocarbons". Tetrahedron Letters. 13 (9): 763–766. doi:10.1016/S0040-4039(01)84432-4.
- ^ Yamamoto, Koji; Harada, Tadashi; Nakazaki, Masao; Naka, Takuo; Kai, Yasushi; Harada, Shigeharu; Kasai, Nobutami (1983). "Synthesis and characterization of [7]circulene". Journal of the American Chemical Society. 105 (24): 7171–7172. doi:10.1021/ja00362a025.
- ^ Koji, Yamamoto; Tadashi, Harada; Yoshio, Okamoto; Hiroaki, Chikamatsu; Masao, Nakazaki; Yasushi, Kai; Takuo, Nakao; Mitsuya, Tanaka; Shigeharu, Harada; Nobutami, Kasai (1988). "Synthesis and molecular structure of [7]circulene". Journal of the American Chemical Society. 110 (11): 3578–3584. doi:10.1021/ja00219a036.
- ^ Yamamoto, K.; Sonobe, H.; Matsubara, H.; Sato, M.; Okamoto, S.; Kitaura, K. (1996). "Convenient New Synthesis of [7]Circulene". Angew. Chem. Int. Ed. Engl. 35: 69–70. doi:10.1002/anie.199600691.
- ^ Extended systems of closed helicene. Synthesis and characterization of [7] and [7.7]-circulene Koji Yamamoto Pure Appl. Chem., Vol. 65, No. 1, pp. 157-163, 1993. Online article
- ^ Bharat, Bhola; Bally, T.; Valente, A.; Cyrański, Michał K.; Dobrzycki, Ł.; Spain, Stephen M.; Rempała, P.; Chin, Matthew R.; King, Benjamin T. (2010). "Quadrannulene: A Nonclassical Fullerene Fragment" (PDF). Angew. Chem. Int. Ed. 49 (2): 399–402. doi:10.1002/anie.200905633. PMID 19957259.
- ^ Feng, C.-N.; Kuo, M.-Y.; Wu, Y.-T. (2013). "Synthesis, Structural Analysis, and Properties of [8]Circulenes". Angew. Chem. Int. Ed. 52 (30): 7791–7794. doi:10.1002/anie.201303875. PMID 23794166.
- ^ Miller, Robert; Duncan, Alexandra; Schneebeli, Severin; Gray, Danielle; Whalley, Adam (24 February 2014). "Synthesis and Structural Data of Tetrabenzo[8]circulene". Chem. Eur. J. 20 (13): 3711. doi:10.1002/chem.201304657. PMC 4371848. PMID 24615957.
- ^ Nakamoto, Youichi; Suzuki, Toshiyasu (2013). "Tetrabenzo[8]circulene: Aromatic Saddles from Negatively Curved Graphene". J. Am. Chem. Soc. 135 (38): 14074–7. doi:10.1021/ja407842z. PMID 24015972.
- ^ Miller, Robert W.; Averill, Summer E.; Van Wyck, Stephen J.; Whalley, Adam C. (2 December 2016). "General Method for the Synthesis of Functionalized Tetrabenzo[8]circulenes". The Journal of Organic Chemistry. 81 (23): 12005. doi:10.1021/acs.joc.6b02244. PMID 27934450.

![Corannulene ([5]circulene)](https://upload.wikimedia.org/wikipedia/commons/thumb/2/23/Corannulene.svg/120px-Corannulene.svg.png)
![Coronene ([6]circulene)](https://upload.wikimedia.org/wikipedia/commons/thumb/0/03/Coronene_200.svg/120px-Coronene_200.svg.png)
![[7]circulene](https://upload.wikimedia.org/wikipedia/commons/thumb/d/d4/-7-circulene.svg/120px--7-circulene.svg.png)
![Kekulene ([12]circulene)](https://upload.wikimedia.org/wikipedia/commons/thumb/d/d9/Kekulene.svg/120px-Kekulene.svg.png)