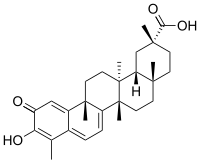
Celastrol
| |
| Names | |
|---|---|
| IUPAC name 3-Hydroxy-9β,13α-dimethyl-2-oxo-24,25,26-trinoroleana-1(10),3,5,7-tetraen-29-oic acid | |
| Systematic IUPAC name (2R,4aS,6aS,12bR,14aS,14bR)-10-Hydroxy-2,4a,6a,9,12b,14a-hexamethyl-11-oxo-1,2,3,4,4a,5,6,6a,11,12b,13,14,14a,14b-tetradecahydropicene-2-carboxylic acid | |
| Other names Tripterine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.164.266 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C29H38O4 | |
| Molar mass | 450.619 g·mol−1 |
| Appearance | Crystalline solid |
| Melting point | 213 °C (415 °F; 486 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Celastrol (tripterine) is a chemical compound isolated from the root extracts of Tripterygium wilfordii (Thunder duke vine) and Tripterygium regelii (Regel's threewingnut). Celastrol is a pentacyclic nortriterpen quinone and belongs to the family of quinone methides. In mice, celastrol is an NR4A1 agonist that alleviates inflammation and induces autophagy.[2] Also in mice, celastrol increase expression of IL1R1, which is the receptor for the cytokine interleukin-1 (IL-1). IL1R1 knock-out mice exposed to celastrol exhibit no leptin-sensitizing or anti-obesity effect.[3]
In in vitro and in vivo animal experiments, celastrol exhibits antibacterial,[4] antioxidant,[5] anti-inflammatory,[6][7] anticancer,[8][9][10][11][12] and insecticidal [13] activities. It has been shown to have obesity-controlling effects in mice by inhibiting negative regulators of leptin.[14][15][16] Celastrol has also shown to possess (by inhibition of NF-κB in the hypothalamus[17]) anti-diabetic effects on diabetic nephropathy and improve whole-body insulin resistance.[18]
Celastrol inhibits IKK-NF-κB signaling via multiple molecular targets: direct inhibition of IKKα and β kinases, inactivation of CDC37 and p23, which are HSP90 chaperone proteins, inhibition of proteasome function and activation of HSF1, which triggers the heat shock response. The available evidence indicates that celastrol bonds covalently to the thiol groups of cysteine residues in its molecular targets.[19]
Celastrol also has demonstrated in vitro inhibitory effects against the carbapenemase of CRE Klebsiella pneumoniae, in combination with thymol, a monoterpene.[20]
References
- ^ Ryu YB, Park SJ, Kim YM, Lee JY, Seo WD, Chang JS, et al. (March 2010). "SARS-CoV 3CLpro inhibitory effects of quinone-methide triterpenes from Tripterygium regelii". Bioorganic & Medicinal Chemistry Letters. 20 (6): 1873–6. doi:10.1016/j.bmcl.2010.01.152. PMC 7127101. PMID 20167482.
- ^ Zhang L, Wang Q, Liu W, Liu F, Ji A, Li Y (2018). "The Orphan Nuclear Receptor 4A1: A Potential New Therapeutic Target for Metabolic Diseases". J Diabetes Res. 2018: 9363461. doi:10.1155/2018/9363461. PMC 6022324. PMID 30013988.
- ^ Feng X, Guan D, Auen T, Choi JW, Salazar Hernández MA, Lee J, et al. (April 2019). "IL1R1 is required for celastrol's leptin-sensitization and antiobesity effects". Nature Medicine. 25 (4): 575–582. doi:10.1038/s41591-019-0358-x. PMC 7158951. PMID 30833749.
- ^ Padilla-Montaño N, de León Guerra L, Moujir L (March 2021). "Antimicrobial Activity and Mode of Action of Celastrol, a Nortriterpen Quinone Isolated from Natural Sources". Foods. 10 (3): 591. doi:10.3390/foods10030591. PMC 7998816. PMID 33799720.
- ^ Allison AC, Cacabelos R, Lombardi VR, Alvarez XA, Vigo C (October 2001). "Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer's disease". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 25 (7): 1341–57. doi:10.1016/S0278-5846(01)00192-0. PMID 11513350. S2CID 21569585.
- ^ Kim DH, Shin EK, Kim YH, Lee BW, Jun JG, Park JH, Kim JK (September 2009). "Suppression of inflammatory responses by celastrol, a quinone methide triterpenoid isolated from Celastrus regelii". European Journal of Clinical Investigation. 39 (9): 819–27. doi:10.1111/j.1365-2362.2009.02186.x. PMID 19549173. S2CID 205291261.
- ^ Venkatesha SH, Yu H, Rajaiah R, Tong L, Moudgil KD (April 2011). "Celastrus-derived celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses". The Journal of Biological Chemistry. 286 (17): 15138–46. doi:10.1074/jbc.M111.226365. PMC 3083183. PMID 21402700.
- ^ Metselaar DS, Meel MH, Benedict B, Waranecki P, Koster J, Kaspers GJL, Hulleman E (November 2019). "Celastrol-induced degradation of FANCD2 sensitizes pediatric high-grade gliomas to the DNA-crosslinking agent carboplatin". EBioMedicine. 50: 81–92. doi:10.1016/j.ebiom.2019.10.062. PMC 6921187. PMID 31735550.
- ^ Lee JH, Choi KJ, Seo WD, Jang SY, Kim M, Lee BW, et al. (March 2011). "Enhancement of radiation sensitivity in lung cancer cells by celastrol is mediated by inhibition of Hsp90". International Journal of Molecular Medicine. 27 (3): 441–6. doi:10.3892/ijmm.2011.601. PMID 21249311.
- ^ Tiedemann RE, Schmidt J, Keats JJ, Shi CX, Zhu YX, Palmer SE, et al. (April 2009). "Identification of a potent natural triterpenoid inhibitor of proteosome chymotrypsin-like activity and NF-kappaB with antimyeloma activity in vitro and in vivo". Blood. 113 (17): 4027–37. doi:10.1182/blood-2008-09-179796. PMC 3952546. PMID 19096011.
- ^ Zhu H, Liu XW, Cai TY, Cao J, Tu CX, Lu W, et al. (August 2010). "Celastrol acts as a potent antimetastatic agent targeting beta1 integrin and inhibiting cell-extracellular matrix adhesion, in part via the p38 mitogen-activated protein kinase pathway". The Journal of Pharmacology and Experimental Therapeutics. 334 (2): 489–99. doi:10.1124/jpet.110.165654. PMID 20472666. S2CID 25854329.
- ^ Byun JY, Kim MJ, Eum DY, Yoon CH, Seo WD, Park KH, et al. (October 2009). "Reactive oxygen species-dependent activation of Bax and poly(ADP-ribose) polymerase-1 is required for mitochondrial cell death induced by triterpenoid pristimerin in human cervical cancer cells". Molecular Pharmacology. 76 (4): 734–44. doi:10.1124/mol.109.056259. PMID 19574249. S2CID 6541041.
- ^ Avilla J, Teixidò A, Velázquez C, Alvarenga N, Ferro E, Canela R (January 2000). "Insecticidal activity of Maytenus species (Celastraceae) nortriterpene quinone methides against codling moth, Cydia pomonella (L.) (Lepidoptera: tortricidae)". Journal of Agricultural and Food Chemistry. 48 (1): 88–92. doi:10.1021/jf990008w. PMID 10637057.
- ^ Kyriakou E, Schmidt S, Dodd GT, et al. Celastrol Promotes Weight Loss in Diet-Induced Obesity by Inhibiting the Protein Tyrosine Phosphatases PTP1B and TCPTP in the Hypothalamus. J Med Chem. 2018;61(24):11144-11157. doi:10.1021/acs.jmedchem.8b01224
- ^ Pfuhlmann K, Schriever SC, Baumann P, Kabra DG, Harrison L, Mazibuko-Mbeje SE, et al. (November 2018). "Celastrol-Induced Weight Loss Is Driven by Hypophagia and Independent From UCP1". Diabetes. 67 (11): 2456–2465. doi:10.2337/db18-0146. PMID 30158241.
- ^ Liu J, Lee J, Salazar Hernandez MA, Mazitschek R, Ozcan U (May 2015). "Treatment of obesity with celastrol". Cell. 161 (5): 999–1011. doi:10.1016/j.cell.2015.05.011. PMC 4768733. PMID 26000480.
- ^ Lee JH, Koo TH, Yoon H, Jung HS, Jin HZ, Lee K, Lee JJ (2006). "Inhibition of NF-κB activation through targeting IκB kinase by celastrol, a quinone methide triterpenoid". Biochemical Pharmacology. 72 (10): 1311–1321. doi:10.1016/j.bcp.2006.08.014. PMID 16984800.
- ^ Kim JE, Lee MH, Nam DH, Song HK, Kang YS, Lee JE, et al. (2013). "Celastrol, an NF-κB inhibitor, improves insulin resistance and attenuates renal injury in db/db mice". PLOS ONE. 8 (4): e62068. Bibcode:2013PLoSO...862068K. doi:10.1371/journal.pone.0062068. PMC 3637455. PMID 23637966.
 This article incorporates text from this source, which is available under the CC BY 4.0 license.
This article incorporates text from this source, which is available under the CC BY 4.0 license.
- ^ Salminen A, Lehtonen M, Paimela T, Kaarniranta K (April 2010). "Celastrol: Molecular targets of Thunder God Vine". Biochem Biophys Res Commun. 394 (3): 439–42. doi:10.1016/j.bbrc.2010.03.050. PMID 20226165.
- ^ Abdel-Halim MS, Askoura M, Mansour B, Yahya G, El-Ganiny AM (27 September 2022). "In vitro activity of celastrol in combination with thymol against carbapenem-resistant Klebsiella pneumoniae isolates". The Journal of Antibiotics. 75: 679–690. doi:10.1038/s41429-022-00566-y. PMC 9640353.
