Caliciviridae
| Caliciviridae | |
|---|---|
| |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Pisuviricota |
| Class: | Pisoniviricetes |
| Order: | Picornavirales |
| Family: | Caliciviridae |
| Genera | |
|
See text | |
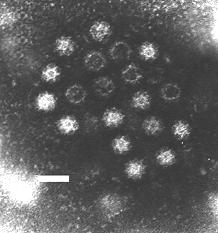
The Caliciviridae are a family of "small round structured" viruses, members of Class IV of the Baltimore scheme. Caliciviridae bear resemblance to enlarged picornavirus and was formerly a separate genus within the picornaviridae.[1] They are positive-sense, single-stranded RNA which is not segmented.[2] Thirteen species are placed in this family, divided among eleven genera.[3] Diseases associated with this family include feline calicivirus (respiratory disease), rabbit hemorrhagic disease virus (often fatal hepatitis), and Norwalk group of viruses (gastroenteritis).[3][4] Caliciviruses naturally infect vertebrates, and have been found in a number of organisms such as humans, cattle, pigs, cats, chickens, reptiles, dolphins and amphibians. The caliciviruses have a simple construction and are not enveloped. The capsid appears hexagonal/spherical and has icosahedral symmetry (T=1[5] or T=3[6][4]) with a diameter of 35–39 nm.[7]
Caliciviruses are not very well studied because until recently, they could not be grown in culture, and they have a very narrow host range and no suitable animal model. However, the recent application of modern genomic technologies has led to an increased understanding of the virus family.[7] A recent isolate from rhesus monkeys—Tulane virus—can be grown in culture, and this system promises to increase understanding of these viruses.[8]
Etymology
Calici- comes from the Latin word Calyx and the Greek word kalyx. The words mean a cup or chalice, a Calix. This comes from the strains having visible cup-shaped depressions.
Taxonomy

The following genera are recognized:[9]
- Bavovirus
- Lagovirus
- Minovirus
- Nacovirus
- Nebovirus
- Norovirus
- Recovirus
- Salovirus
- Sapovirus
- Valovirus
- Vesivirus
A number of other caliciviruses remain unclassified, including the chicken calicivirus.
Virology

Life cycle
Viral replication is cytoplasmic. Entry into the host cell is achieved by attachment to host receptors, which mediate endocytosis. Replication follows the positive-stranded RNA virus replication model. Positive-stranded RNA virus transcription is the method of transcription. Translation takes place by leaky scanning, and RNA termination-reinitiation. Vertebrates serve as the natural host. Transmission routes are fecal-oral.[4]
Human disease
History
Establishing the viral etiology took many decades due to the difficulty of growing the virus in cell culture. In the 1940s and 1950s in the United States and Japan, caliciviridae could not be grown in culture, but as an experiment bacterial free filtrate of diarrhea was given to volunteers to check if viruses were present in volunteers' stool.[10] These experiments demonstrated that nonbacterial, filterable agents had the capability of causing enteric disease in humans.[10] In 1968, an outbreak at a Norwalk elementary school (e.g. Norwalk virus) in Ohio led to stool samples again being given to volunteers and serially passaged to other people. Finally, in 1972, Kapikian and his colleagues isolated the Norwalk virus from volunteers using immune electron microscopy, a process that involves looking directly at antibody-antigen complexes.[10] The classification of this one Norwalk virus strain served as the prototype for other species and small round structured viruses later known as Norovirus.[10]
Animal viruses
Rabbit hemorrhagic disease virus is a pathogen of rabbits that causes major problems throughout the world where rabbits are reared for food and clothing, make a significant contribution to ecosystem ecology, and where they support valued wildlife as a food source.[7]
Uses
Australia and New Zealand, in an effort to control their rabbit populations, have intentionally spread rabbit calicivirus.
References
- ^ "Caliciviridae - Caliciviridae - Positive-sense RNA Viruses - ICTV". talk.ictvonline.org. Retrieved 12 March 2021.
- ^ Vinjé, J; Estes, MK; Esteves, P; Green, KY; Katayama, K; Knowles, NJ; L'Homme, Y; Martella, V; Vennema, H; White, PA; ICTV Report Consortium (November 2019). "ICTV Virus Taxonomy Profile: Caliciviridae". The Journal of General Virology. 100 (11): 1469–1470. doi:10.1099/jgv.0.001332. PMC 7011698. PMID 31573467.
- ^ a b "ICTV Report Caliciviridae".
- ^ a b c "Viral Zone". ExPASy. Retrieved 15 June 2015.
- ^ T=1
- ^ T=3
- ^ a b c Hansman, GS, ed. (2010). Caliciviruses: Molecular and Cellular Virology. Caister Academic Press. ISBN 978-1-904455-63-9.
- ^ Yu G, Zhang D, Guo F, Tan M, Jiang X, Jiang W (2013). "Cryo-EM structure of a novel calicivirus, Tulane Virus". PLoS One 8(3):e59817. doi:10.1371/journal.pone.0059817
- ^ "Virus Taxonomy: 2019 Release". talk.ictvonline.org. International Committee on Taxonomy of Viruses. Retrieved 30 April 2020.
- ^ a b c d Themes, U. F. O. (11 August 2016). "Caliciviridae: The Noroviruses". Basicmedical Key. Retrieved 12 March 2021.
External links
- ICTV Report: Caliciviridae
- Caliciviridae description page from the International Committee on Taxonomy of Viruses site
- MicrobiologyBytes: Caliciviruses
- Human caliciviruses
- Stanford University
- Virus Pathogen Database and Analysis Resource (ViPR): Caliciviridae
- Viralzone: Caliciviridae
- 3D macromolecular structures of Caliciviridae from the EM Data Bank(EMDB)
- ICTV
