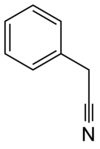
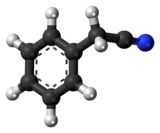
Benzyl cyanide
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name Phenylacetonitrile[1] | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.004.919 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H7N | |
| Molar mass | 117.15 g/mol |
| Appearance | Colorless oily liquid |
| Density | 1.015 g/cm3 |
| Melting point | −24 °C (−11 °F; 249 K) |
| Boiling point | 233 to 234 °C (451 to 453 °F; 506 to 507 K) |
| -76.87·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Benzyl cyanide (abbreviated BnCN) is an organic compound with the chemical formula C6H5CH2CN. This colorless oily aromatic liquid is an important precursor to numerous compounds in organic chemistry.[2] It is also an important pheromone in certain species.[3]
Preparation
Benzyl cyanide can be produced via Kolbe nitrile synthesis between benzyl chloride and sodium cyanide[4] and by oxidative decarboxylation of phenylalanine.[5]
Benzyl cyanides can also be prepared by arylation of silyl-substituted acetonitrile.[6]
Reactions
Benzyl cyanide undergoes many reactions characteristic of nitriles. It can be hydrolyzed to give phenylacetic acid[7] or it can be used in the Pinner reaction to yield phenylacetic acid esters.[8] Hydrogenation gives β-phenethylamine.[9]
The compound contains an "active methylene unit". Bromination occurs gives PhCHBrCN.[10] A variety of base-induced reactions result in the formation of new carbon-carbon bonds.[11][12][13]
Uses
Benzyl cyanide is used as a solvent[14] and as a starting material in the synthesis of fungicides (e.g. Fenapanil),[15] fragrances (phenethyl alcohol), antibiotics,[2] and other pharmaceuticals. The partial hydrolysis of BnCN results in 2-phenylacetamide.[16]
Pharmaceuticals
Benzyl cyanide is a useful precursor to numerous pharmaceuticals. Examples include: [17]
- Antiarrhythmics (e.g. disopyramide)[17]
- Antidepressants: E.g. Milnacipran & Lomevactone
- Antihistamines (e.g. levocabastine (para-fluoro),[17][18] Pheniramine & Azatadine.
- Antitussives (e.g. isoaminile, oxeladin, butethamate, pentapiperide, and pentoxyverine)[19]
- Diuretics (e.g. triamterene)[20]
- Hypnotics (e.g. alonimid and phenobarbital)[17][21] & Phenglutarimide
- Spasmolytics (e.g. pentapiperide and drofenine)[17][22]
- Stimulants (e.g. methylphenidate)[17]
- Opioids (e.g. ethoheptazine, pethidine, and phenoperidine)[17] & methadone
Regulation
Because benzyl cyanide is a useful precursor to numerous drugs with recreational use potential, many countries strictly regulate the compound.
United States
Benzyl cyanide is regulated in the United States as a DEA List I chemical.
China
Benzyl cyanide is regulated in People's Republic of China as a Class III drug precursor since 7 June 2021.[23]
Safety
Benzyl cyanide, like related benzyl derivatives, is an irritant to the skin and eyes.[2]
See also
References
- ^ a b Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 16. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ a b c Pollak, Peter; Romeder, Gérard; Hagedorn, Ferdinand; Gelbke, Heinz-Peter (2000). "Nitriles". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a17_363. ISBN 3527306730.
- ^ "Toxin Protects Migratory Locusts from Cannibalism". Max Planck Society. 4 May 2023. Retrieved 8 December 2024.
- ^ Adams, Roger; Thal, A. F. (1922). "Benzyl cyanide". Organic Syntheses. 2: 9. doi:10.15227/orgsyn.002.0009.
- ^ Hiegel, Gene; Lewis, Justin; Bae, Jason (2004). "Conversion of α-Amino Acids into Nitriles by Oxidative Decarboxylation with Trichloroisocyanuric Acid". Synthetic Communications. 34 (19): 3449–3453. doi:10.1081/SCC-200030958. S2CID 52208189.
- ^ Wu, Lingyun; Hartwig, John F. (2005). "Mild Palladium-Catalyzed Selective Monoarylation of Nitriles". Journal of the American Chemical Society. 127 (45): 15824–15832. doi:10.1021/ja053027x. PMID 16277525.
- ^ Adams, Roger; Thal, A. F. (1922). "Phenylacetic acid". Organic Syntheses. 2: 59. doi:10.15227/orgsyn.002.0059.
- ^ Adams, Roger; Thal, A. F. (1922). "Ethyl Phenylacetate". Organic Syntheses. 2: 27. doi:10.15227/orgsyn.002.0027.
- ^ Robinson, John C. Jr.; Snyder, H. R. (1943). "β-Phenylethylamine". Organic Syntheses. 23: 71. doi:10.15227/orgsyn.023.0071.
- ^ Robb, C. M.; Schultz, E. M. (1948). "Diphenylacetonitrile". Organic Syntheses. 28: 55. doi:10.15227/orgsyn.028.0055.
- ^ Makosza, M.; Jonczyk, A (1976). "Phase-Transfer Alkylation of Nitriles: 2-Phenylbutyronitrile". Organic Syntheses. 55: 91. doi:10.15227/orgsyn.055.0091.
- ^ Itoh, Masumi; Hagiwara, Daijiro; Kamiya, Takashi (1988). "New Reagent for tert-Butoxycarbonylation: 2-tert-Butoxycarbonyloxyimino-2-phenylacetonitrile". Organic Syntheses. 6: 199. doi:10.15227/orgsyn.059.0095.
- ^ Wawzonek, Stanley; Smolin, Edwin M. (1955). "α-Phenylcinnamonitrile". Organic Syntheses. 3: 715. doi:10.15227/orgsyn.029.0083.
- ^ Bien, Hans-Samuel; Stawitz, Josef; Wunderlich, Klaus (2000). "Anthraquinone Dyes and Intermediates". Ullmann's Encyclopedia of Industrial Chemistry: 29. doi:10.1002/14356007.a02_355. ISBN 3527306730.
- ^ Ackermann, Peter; Margot, Paul; Müller, Franz (2000). "Fungicides, Agricultural". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a12_085. ISBN 3527306730.
- ^ "PHENYLACETAMIDE". Organic Syntheses. 32: 92. 1952. doi:10.15227/orgsyn.032.0092. ISSN 0078-6209.
- ^ a b c d e f g William Andrew Publishing (2008). Pharmaceutical Manufacturing Encyclopedia (3rd ed.). Norwich, NY: Elsevier Science. pp. 182, 936, 1362, 1369, 1505, 2036, 2157, 2259, 2554, 2620, 2660, 2670, 2924, 3032, & 3410. ISBN 9780815515265.
- ^ Berkoff, Charles E.; Rivard, Donald E.; Kirkpatrick, David; Ives, Jeffrey L. (1980). "The Reductive Decyanation of Nitriles by Alkali Fusion". Synthetic Communications. 10 (12): 939–945. doi:10.1080/00397918008061855.
- ^ Bub, Oskar; Friedrich, Ludwig (2000). "Cough Remedies". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a08_013. ISBN 3527306730.
- ^ Hropot, Max; Lang, Hans-Jochen (2000). "Diuretics". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a09_029. ISBN 3527306730.
- ^ Furniss, Brian; Hannaford, Antony; Smith, Peter & Tatchell, Austin (1996). Vogel's Textbook of Practical Organic Chemistry 5th Ed. London: Longman Science & Technical. pp. 1174–1179. ISBN 9780582462366.
- ^ Bungardt, Edwin; Mutschler, Ernst (2000). "Spasmolytics". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a24_515. ISBN 3527306730.
- ^ "国务院办公厅关于同意将α-苯乙酰乙酸甲酯等6种物质列入易制毒化学品品种目录的函" (in Simplified Chinese). The State Council - The People's Republic of China. 7 June 2021. Retrieved 11 October 2021.
