Benzylamine

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name Phenylmethanamine | |
| Other names α-Aminotoluene Benzyl amine Phenylmethylamine | |
| Identifiers | |
3D model (JSmol) |
|
| 741984 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.002.595 |
| EC Number |
|
| 49783 | |
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2735 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H9N | |
| Molar mass | 107.156 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | weak, ammonia-like |
| Density | 0.981 g/mL[1] |
| Melting point | 10 °C (50 °F; 283 K)[2] |
| Boiling point | 185 °C (365 °F; 458 K)[2] |
| Miscible[2] | |
| Solubility | miscible in ethanol, diethyl ether very soluble in acetone soluble in benzene, chloroform |
| Acidity (pKa) | 9.34[3] |
| Basicity (pKb) | 4.66 |
| -75.26·10−6 cm3/mol | |
Refractive index (nD) |
1.543 |
| Structure | |
| 1.38 D | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Flammable and corrosive |
| GHS labelling: | |
 
| |
| Danger | |
| H302, H312, H314 | |
| P260, P264, P270, P280, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P322, P330, P363, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 65 °C (149 °F; 338 K)[2][1] |
| Safety data sheet (SDS) | Fischer Scientific |
| Related compounds | |
Related amines |
aniline |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Benzylamine, also known as phenylmethylamine, is an organic chemical compound with the condensed structural formula C6H5CH2NH2 (sometimes abbreviated as PhCH2NH2 or BnNH2). It consists of a benzyl group, C6H5CH2, attached to an amine functional group, NH2. This colorless water-soluble liquid is a common precursor in organic chemistry and used in the industrial production of many pharmaceuticals. The hydrochloride salt was used to treat motion sickness on the Mercury-Atlas 6 mission in which NASA astronaut John Glenn became the first American to orbit the Earth.
Manufacturing
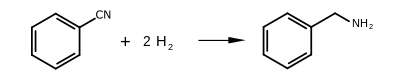
Benzylamine can be produced by several methods, the main industrial route being the reaction of benzyl chloride and ammonia. It is also produced by the reduction of benzonitrile and reductive amination of benzaldehyde, both done over Raney nickel.[4]
It was first produced accidentally by Rudolf Leuckart in the reaction of benzaldehyde with formamide in a process now known as the Leuckart reaction.[5]
Biochemistry
Benzylamine occurs biologically from the action of the N-substituted formamide deformylase enzyme, which is produced by Arthrobacter pascens bacteria.[6] This hydrolase catalyses the conversion of N-benzylformamide into benzylamine with formate as a by-product.[7] Benzylamine is degraded biologically by the action of the monoamine oxidase B enzyme,[8] resulting in benzaldehyde.[9]
Uses
Benzylamine is used as a masked source of ammonia, since after N-alkylation, the benzyl group can be removed by hydrogenolysis:[10]
- C6H5CH2NH2 + 2 RBr → C6H5CH2NR2 + 2 HBr
- C6H5CH2NR2 + H2 → C6H5CH3 + R2NH
Typically a base is employed in the first step to absorb the HBr (or related acid for other kinds of alkylating agents).
Benzylamine reacts with acetyl chloride to form N-benzylacetamide.
Isoquinolines can be prepared from benzylamine and glyoxal acetal by an analogous approach known as the Schlittler-Müller modification to the Pomeranz–Fritsch reaction. This modification can also be used for preparing substituted isoquinolines.[11]
Benzylamine is used in the manufacture of other pharmaceuticals, including alniditan,[12] lacosamide,[13][14] moxifloxacin,[15] and nebivolol.[16]
Benzylamine is also used to manufacture the military explosive hexanitrohexaazaisowurtzitane (HNIW), which is superior to older nitroamine high explosives like HMX and RDX. Illustrating the debenzylation tendency of benzylamines, four of the benzyl groups are removed from hexabenzylhexaazaisowurtzitane by hydrogenolysis catalysed by palladium on carbon.[17]
Pharmacology and derivatives
Benzylamine has been found to act as a monoamine oxidase inhibitor (MAOI), including of both monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B).[18]
A derivative, pargyline (N-Methyl-N-propargylbenzylamine), is an MAOI that has been used pharmaceutically as an antihypertensive agent and antidepressant.[19] α-Methylbenzylamine is an MAOI, inhibiting both MAO-A and MAO-B, as well.[18]
Another derivative, α,N-DMMDBA (MDM1EA; α,N-dimethyl-3,4-methylenedioxybenzylamine), partially substitutes for MDMA at high doses in drug discrimination tests in rats.[20][21] Benzylamine is also similar in structure to benzylpiperazine (BZP), which is a monoamine releasing agent and psychostimulant.[22] However, both benzylamine and α-methylbenzylamine have been found to be inactive as norepinephrine releasing agents.[23]
Salts
The hydrochloride salt of benzylamine, C6H5CH2NH3Cl or C6H5CH2NH2·HCl,[24] is prepared by reacting benzylamine with hydrochloric acid, and can be used in treating motion sickness. NASA astronaut John Glenn was issued with benzylamine hydrochloride for this purpose for the Mercury-Atlas 6 mission.[25] The cation in this salt is called benzylammonium and is a moiety found in pharmaceuticals such as the anthelmintic agent bephenium hydroxynaphthoate, used in treating ascariasis.[26]
Other derivatives of benzylamine and its salts have been shown to have anti-emetic properties, including those with the N-(3,4,5-trimethoxybenzoyl)benzylamine moiety.[27] Commercially available motion-sickness agents including cinnarizine and meclizine are derivatives of benzylamine.
Other benzylamines
1-Phenylethylamine is a methylated benzylamine derivative that is chiral; enantiopure forms are obtained by resolving racemates. Its racemic form is sometimes known as (±)-α-methylbenzylamine.[28] Both benzylamine and 1-phenylethylamine form stable ammonium salts and imines due to their relatively high basicity.
Safety and environment
Benzylamine exhibits modest oral toxicity in rats with LD50 of 1130 mg/kg. It is readily biodegraded.[4]
References
- ^ a b "Benzylamine". Sigma-Aldrich. Retrieved 28 December 2015.
- ^ a b c d Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ Hall, H. K. (1957). "Correlation of the Base Strengths of Amines". J. Am. Chem. Soc. 79 (20): 5441–5444. doi:10.1021/ja01577a030.
- ^ a b Heuer, L. (2006). "Benzylamines". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:10.1002/14356007.a04_009.pub2. ISBN 3527306730.
- ^ Moore, Maurice L. (2011). "The Leuckart Reaction". Organic Reactions. pp. 301–330. doi:10.1002/0471264180.or005.07. ISBN 978-0-471-26418-7.
- ^ Schomburg, D.; Schomburg, I.; Chang, A., eds. (2009). "3.5.1.91 N-substituted formamide deformylase". Class 3 Hydrolases: EC 3.4.22–3.13. Springer Handbook of Enzymes (2nd ed.). Springer Science & Business Media. pp. 376–378. ISBN 9783540857051.
- ^ Fukatsu, H.; Hashimoto, Y.; Goda, M.; Higashibata, H.; Kobayashi, M. (2004). "Amine-synthesizing enzyme N-substituted formamide deformylase: screening, purification, characterization, and gene cloning". Proc. Natl. Acad. Sci. 101 (38): 13726–13731. Bibcode:2004PNAS..10113726F. doi:10.1073/pnas.0405082101. PMC 518824. PMID 15358859.
- ^ "MAOB: Monoamine oxidase B – Homo sapiens". National Center for Biotechnology Information. 6 December 2015. Retrieved 29 December 2015.
- ^ Tipton, K. F.; Boyce, S.; O'Sullivan, J.; Davey, G. P.; Healy, J. (2004). "Monoamine oxidases: Certainties and uncertainties". Curr. Med. Chem. 11 (15): 1965–1982. doi:10.2174/0929867043364810. PMID 15279561.
- ^ Gatto, V. J.; Miller, S. R.; Gokel, G. W. (1993). "4,13-Diaza-18-Crown-6". Organic Syntheses; Collected Volumes, vol. 8, p. 152. (example of alklylation of benzylamine followed by hydrogenolysis).
- ^ Li, J. J. (2014). "Schlittler–Müller modification". Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications (5th ed.). Springer. p. 492. ISBN 9783319039794.
- ^ Lommen, G.; De Bruyn, M.; Schroven, M.; Verschueren, W.; Janssens, W.; Verrelst, J.; Leysen, J. (1995). "The discovery of a series of new non-indole 5HT1D agonists". Bioorg. Med. Chem. Lett. 5 (22): 2649–2654. doi:10.1016/0960-894X(95)00473-7.
- ^ Choi, D.; Stables, J. P.; Kohn, H. (1996). "Synthesis and anticonvulsant activities of N-Benzyl-2-acetamidopropionamide derivatives". J. Med. Chem. 39 (9): 1907–1916. doi:10.1021/jm9508705. PMID 8627614.
- ^ Morieux, P.; Stables, J. P.; Kohn, H. (2008). "Synthesis and anticonvulsant activities of N-benzyl-(2R)-2-acetamido-3-oxysubstituted propionamide derivatives". Bioorg. Med. Chem. 16 (19): 8968–8975. doi:10.1016/j.bmc.2008.08.055. PMC 2701728. PMID 18789868.
- ^ Peterson, U. (2006). "Quinolone Antibiotics: The Development of Moxifloxacin". In IUPAC; Fischer, J.; Ganellin, C. R. (eds.). Analogue-based Drug Discovery. John Wiley & Sons. pp. 338–342. ISBN 9783527607495.
- ^ US patent 4654362, Van Lommen, G. R. E.; De Bruyn, M. F. L. & Schroven, M. F. J., "Derivatives of 2,2'-iminobisethanol", published 1987-03-31, assigned to Janssen Pharmaceutica, N.V.. Full text
- ^ Nair, U. R.; Sivabalan, R.; Gore, G. M.; Geetha, M.; Asthana, S. N.; Singh, H. (2005). "Hexanitrohexaazaisowurtzitane (CL-20) and CL-20-based formulations (review)". Combust. Explos. Shock Waves. 41 (2): 121–132. doi:10.1007/s10573-005-0014-2. S2CID 95545484.
- ^ a b Nakagawasai O, Arai Y, Satoh SE, Satoh N, Neda M, Hozumi M, Oka R, Hiraga H, Tadano T (January 2004). "Monoamine oxidase and head-twitch response in mice. Mechanisms of alpha-methylated substrate derivatives". Neurotoxicology. 25 (1–2): 223–232. Bibcode:2004NeuTx..25..223N. doi:10.1016/S0161-813X(03)00101-3. PMID 14697897.
- ^ Ho BT (June 1972). "Monoamine oxidase inhibitors". J Pharm Sci. 61 (6): 821–837. doi:10.1002/jps.2600610602. PMID 4558257.
- ^ Shulgin, A.; Manning, T.; Daley, P.F. (2011). The Shulgin Index, Volume One: Psychedelic Phenethylamines and Related Compounds. Vol. 1. Berkeley: Transform Press. ISBN 978-0-9630096-3-0.
- ^ Bronson ME, Barrios-Zambrano L, Jiang W, Clark CR, DeRuiter J, Newland MC (December 1994). "Behavioral and developmental effects of two 3,4-methylenedioxymethamphetamine (MDMA) derivatives". Drug Alcohol Depend. 36 (3): 161–166. doi:10.1016/0376-8716(94)90141-4. PMID 7889806.
- ^ Gee, Paul; Schep, Leo J. (2022). "1-Benzylpiperazine and other piperazine-based stimulants". Novel Psychoactive Substances. Elsevier. pp. 301–332. doi:10.1016/b978-0-12-818788-3.00009-7. ISBN 978-0-12-818788-3.
- ^ Biel, J. H.; Bopp, B. A. (1978). "Amphetamines: Structure-Activity Relationships". Stimulants. Boston, MA: Springer US. p. 1–39. doi:10.1007/978-1-4757-0510-2_1. ISBN 978-1-4757-0512-6.
The β-phenethylamine skeleton is a critical feature of the molecule since either increasing or decreasing the number of carbons between the phenyl ring and the nitrogen reduced or abolished the activity. Both the γ-phenylpropylamines (e.g., 1-phenyl-3-aminobutane, γ-phenylpropylamine, γ-phenyl-N,N-dimethylpropylamine) and the benzylamines (e.g., α-methylbenzylamine, N,N-diethylbenzylamine, benzylamine) were found to be inactive as releasers of norepinephrine (Daly et al., 1966).
- ^ "Benzylamine hydrochloride". Sigma-Aldrich. Retrieved 28 December 2015.
- ^ Swenson, L. S.; Grimwood, J. M.; Alexander, C. C. "13: Mercury Mission Accomplished (13.1 Preparing a Man to Orbit)". This New Ocean: A History of Project Mercury. nasa.gov. pp. 413–418.
- ^ Hellgren, U.; Ericsson, Ö.; Aden Abdi, Y.; Gustafsson, L. L. (2003). "Bephenium hydroxynaphthoate". Handbook of Drugs for Tropical Parasitic Infections (2nd ed.). CRC Press. pp. 33–35. ISBN 9780203211519.
- ^ US patent 2879293, Sidney, T. & Goldberg, M. W., "Benzylamine derivatives", published 1959-03-24, issued 1959-03-24, assigned to Hoffmann La Roche. Full text
- ^ PubChem Public Chemical Database (26 December 2015). "1-Phenylethylamine". National Center for Biotechnology Information. Retrieved 29 December 2015.