Benzoic acid
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Benzoic acid[1] | |||
| Systematic IUPAC name Benzenecarboxylic acid | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| 3DMet | |||
| 636131 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.562 | ||
| EC Number |
| ||
| E number | E210 (preservatives) | ||
| 2946 | |||
| KEGG | |||
| MeSH | benzoic+acid | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C7H6O2 | |||
| Molar mass | 122.123 g/mol | ||
| Appearance | Colorless crystalline solid | ||
| Odor | Faint, pleasant odor | ||
| Density | 1.2659 g/cm3 (15 °C) 1.0749 g/cm3 (130 °C)[2] | ||
| Melting point | 122 °C (252 °F; 395 K)[7] | ||
| Boiling point | 250 °C (482 °F; 523 K)[7] | ||
| 1.7 g/L (0 °C) 2.7 g/L (18 °C) 3.44 g/L (25 °C) 5.51 g/L (40 °C) 21.45 g/L (75 °C) 56.31 g/L (100 °C)[2][3] | |||
| Solubility | Soluble in acetone, benzene, CCl4, CHCl3, alcohol, ethyl ether, hexane, phenyls, liquid ammonia, acetates | ||
| Solubility in methanol | 30 g/100 g (−18 °C) 32.1 g/100 g (−13 °C) 71.5 g/100 g (23 °C)[2] | ||
| Solubility in ethanol | 25.4 g/100 g (−18 °C) 47.1 g/100 g (15 °C) 52.4 g/100 g (19.2 °C) 55.9 g/100 g (23 °C)[2] | ||
| Solubility in acetone | 54.2 g/100 g (20 °C)[2] | ||
| Solubility in olive oil | 4.22 g/100 g (25 °C)[2] | ||
| Solubility in 1,4-dioxane | 55.3 g/100 g (25 °C)[2] | ||
| log P | 1.87 | ||
| Vapor pressure | 0.16 Pa (25 °C) 0.19 kPa (100 °C) 22.6 kPa (200 °C)[4] | ||
| Acidity (pKa) | |||
| −70.28·10−6 cm3/mol | |||
Refractive index (nD) |
1.5397 (20 °C) 1.504 (132 °C)[2] | ||
| Viscosity | 1.26 mPa (130 °C) | ||
| Structure | |||
| Monoclinic | |||
| Planar | |||
| 1.72 D in dioxane | |||
| Thermochemistry | |||
Heat capacity (C) |
146.7 J/mol·K[4] | ||
Std molar entropy (S⦵298) |
167.6 J/mol·K[2] | ||
Std enthalpy of formation (ΔfH⦵298) |
−385.2 kJ/mol[2] | ||
Std enthalpy of combustion (ΔcH⦵298) |
−3228 kJ/mol[4] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
Irritant | ||
| GHS labelling: | |||
  [8] [8]
| |||
| Danger | |||
| H318, H335[8] | |||
| P261, P280, P305+P351+P338[8] | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 121.5 °C (250.7 °F; 394.6 K)[7] | ||
| 571 °C (1,060 °F; 844 K)[7] | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
1700 mg/kg (rat, oral) | ||
| Safety data sheet (SDS) | JT Baker | ||
| Related compounds | |||
Other cations |
Sodium benzoate, Potassium benzoate | ||
Related carboxylic acids |
Hydroxybenzoic acids Aminobenzoic acids, Nitrobenzoic acids, Phenylacetic acid | ||
Related compounds |
Benzaldehyde, Benzyl alcohol, Benzoyl chloride, Benzylamine, Benzamide, Benzonitrile | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Benzoic acid (/bɛnˈzoʊ.ɪk/) is a white (or colorless) solid organic compound with the formula C6H5COOH, whose structure consists of a benzene ring (C6H6) with a carboxyl (−C(=O)OH) substituent. The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn," which is used for benzyl), thus benzoic acid is also denoted as BzOH, since the benzoyl group has the formula –C6H5CO. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, which was for a long time its only source.
Benzoic acid occurs naturally in many plants[9] and serves as an intermediate in the biosynthesis of many secondary metabolites. Salts of benzoic acid are used as food preservatives. Benzoic acid is an important precursor for the industrial synthesis of many other organic substances. The salts and esters of benzoic acid are known as benzoates (/ˈbɛnzoʊ.eɪt/).
History
Benzoic acid was discovered in the sixteenth century. The dry distillation of gum benzoin was first described by Nostradamus (1556), and then by Alexius Pedemontanus (1560) and Blaise de Vigenère (1596).[10]
Justus von Liebig and Friedrich Wöhler determined the composition of benzoic acid.[11] These latter also investigated how hippuric acid is related to benzoic acid.
In 1875 Salkowski discovered the antifungal properties of benzoic acid, which was used for a long time in the preservation of benzoate-containing cloudberry fruits.[12][disputed – discuss]
Production
Industrial preparations
Benzoic acid is produced commercially by partial oxidation of toluene with oxygen. The process is catalyzed by cobalt or manganese naphthenates. The process uses abundant materials, and proceeds in high yield.[13]
The first industrial process involved the reaction of benzotrichloride (trichloromethyl benzene) with calcium hydroxide in water, using iron or iron salts as catalyst. The resulting calcium benzoate is converted to benzoic acid with hydrochloric acid. The product contains significant amounts of chlorinated benzoic acid derivatives. For this reason, benzoic acid for human consumption was obtained by dry distillation of gum benzoin. Food-grade benzoic acid is now produced synthetically.
Laboratory synthesis
Benzoic acid is cheap and readily available, so the laboratory synthesis of benzoic acid is mainly practiced for its pedagogical value. It is a common undergraduate preparation.
Benzoic acid can be purified by recrystallization from water because of its high solubility in hot water and poor solubility in cold water. The avoidance of organic solvents for the recrystallization makes this experiment particularly safe. This process usually gives a yield of around 65%.[14]
By hydrolysis
Like other nitriles and amides, benzonitrile and benzamide can be hydrolyzed to benzoic acid or its conjugate base in acid or basic conditions.
From Grignard reagent
Bromobenzene can be converted to benzoic acid by "carboxylation" of the intermediate phenylmagnesium bromide.[15] This synthesis offers a convenient exercise for students to carry out a Grignard reaction, an important class of carbon–carbon bond forming reaction in organic chemistry.[16][17][18][19][20]
Oxidation of benzyl compounds
Benzyl alcohol[21] and benzyl chloride and virtually all benzyl derivatives are readily oxidized to benzoic acid.
Uses
Benzoic acid is mainly consumed in the production of phenol by oxidative decarboxylation at 300−400 °C:[22]
The temperature required can be lowered to 200 °C by the addition of catalytic amounts of copper(II) salts. The phenol can be converted to cyclohexanol, which is a starting material for nylon synthesis.
Precursor to plasticizers
Benzoate plasticizers, such as the glycol-, diethyleneglycol-, and triethyleneglycol esters, are obtained by transesterification of methyl benzoate with the corresponding diol.[22] These plasticizers, which are used similarly to those derived from terephthalic acid ester, represent alternatives to phthalates.[22]
Precursor to sodium benzoate and related preservatives
Benzoic acid and its salts are used as food preservatives, represented by the E numbers E210, E211, E212, and E213. Benzoic acid inhibits the growth of mold, yeast[23] and some bacteria. It is either added directly or created from reactions with its sodium, potassium, or calcium salt. The mechanism starts with the absorption of benzoic acid into the cell. If the intracellular pH changes to 5 or lower, the anaerobic fermentation of glucose through phosphofructokinase is decreased by 95%. The efficacy of benzoic acid and benzoate is thus dependent on the pH of the food.[24] Benzoic acid, benzoates and their derivatives are used as preservatives for acidic foods and beverages such as citrus fruit juices (citric acid), sparkling drinks (carbon dioxide), soft drinks (phosphoric acid), pickles (vinegar) and other acidified foods.
Typical concentrations of benzoic acid as a preservative in food are between 0.05 and 0.1%. Foods in which benzoic acid may be used and maximum levels for its application are controlled by local food laws.[25][26]
Concern has been expressed that benzoic acid and its salts may react with ascorbic acid (vitamin C) in some soft drinks, forming small quantities of carcinogenic benzene.[27]
Medicinal
Benzoic acid is a constituent of Whitfield's ointment which is used for the treatment of fungal skin diseases such as ringworm and athlete's foot.[28][29] As the principal component of gum benzoin, benzoic acid is also a major ingredient in both tincture of benzoin and Friar's balsam. Such products have a long history of use as topical antiseptics and inhalant decongestants.
Benzoic acid was used as an expectorant, analgesic, and antiseptic in the early 20th century.[30]
Niche and laboratory uses
In teaching laboratories, benzoic acid is a common standard for calibrating a bomb calorimeter.[31]
Biology and health effects
Benzoic acid occurs naturally as do its esters in many plant and animal species. Appreciable amounts are found in most berries (around 0.05%). Ripe fruits of several Vaccinium species (e.g., cranberry, V. vitis macrocarpon; bilberry, V. myrtillus) contain as much as 0.03–0.13% free benzoic acid. Benzoic acid is also formed in apples after infection with the fungus Nectria galligena. Among animals, benzoic acid has been identified primarily in omnivorous or phytophageous species, e.g., in viscera and muscles of the rock ptarmigan (Lagopus muta) as well as in gland secretions of male muskoxen (Ovibos moschatus) or Asian bull elephants (Elephas maximus).[32] Gum benzoin contains up to 20% of benzoic acid and 40% benzoic acid esters.[33]
In terms of its biosynthesis, benzoate is produced in plants from cinnamic acid.[34] A pathway has been identified from phenol via 4-hydroxybenzoate.[35]
Reactions
Reactions of benzoic acid can occur at either the aromatic ring or at the carboxyl group.
Aromatic ring
Electrophilic aromatic substitution reaction will take place mainly in 3-position due to the electron-withdrawing carboxylic group; i.e. benzoic acid is meta directing.[36]
Carboxyl group
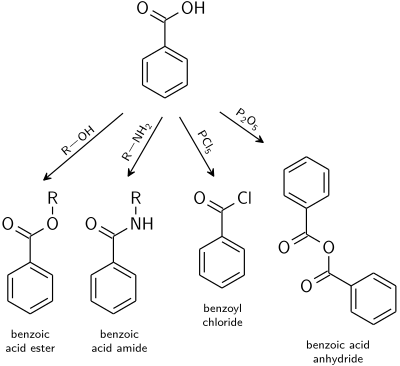
Reactions typical for carboxylic acids apply also to benzoic acid.[22]
- Benzoate esters are the product of the acid catalysed reaction with alcohols.
- Benzoic acid amides are usually prepared from benzoyl chloride.
- Dehydration to benzoic anhydride is induced with acetic anhydride or phosphorus pentoxide.
- Highly reactive acid derivatives such as acid halides are easily obtained by mixing with halogenation agents like phosphorus chlorides or thionyl chloride.
- Orthoesters can be obtained by the reaction of alcohols under acidic water free conditions with benzonitrile.
- Reduction to benzaldehyde and benzyl alcohol is possible using DIBAL-H, LiAlH4 or sodium borohydride.
- Decarboxylation to benzene may be effected by heating in quinoline in the presence of copper salts. Hunsdiecker decarboxylation can be achieved by heating the silver salt.
Safety and mammalian metabolism
It is excreted as hippuric acid.[37] Benzoic acid is metabolized by butyrate-CoA ligase into an intermediate product, benzoyl-CoA,[38] which is then metabolized by glycine N-acyltransferase into hippuric acid.[39] Humans metabolize toluene which is also excreted as hippuric acid.[40]
For humans, the World Health Organization's International Programme on Chemical Safety (IPCS) suggests a provisional tolerable intake would be 5 mg/kg body weight per day.[32] Cats have a significantly lower tolerance against benzoic acid and its salts than rats and mice. Lethal dose for cats can be as low as 300 mg/kg body weight.[41] The oral LD50 for rats is 3040 mg/kg, for mice it is 1940–2263 mg/kg.[32]
In Taipei, Taiwan, a city health survey in 2010 found that 30% of dried and pickled food products had benzoic acid.[42]
See also
- Niacin – Organic compound and a form of vitamin B3
References
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 745. doi:10.1039/9781849733069-00648. ISBN 978-0-85404-182-4.
- ^ a b c d e f g h i j "benzoic acid". chemister.ru. Retrieved 24 October 2018.
- ^ Seidell, Atherton; Linke, William F. (1952). Solubilities of Inorganic and Organic Compounds. Van Nostrand.
- ^ a b c Benzoic acid in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD) (retrieved 2014-05-23)
- ^ Harris, Daniel (2010). Quantitative Chemical Analysis (8 ed.). New York: W. H. Freeman and Company. pp. AP12. ISBN 9781429254366.
- ^ Olmstead, William N.; Bordwell, Frederick G. (1980). "Ion-pair association constants in dimethyl sulfoxide". The Journal of Organic Chemistry. 45 (16): 3299–3305. doi:10.1021/jo01304a033.
- ^ a b c d Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ a b c Sigma-Aldrich Co., Benzoic acid. Retrieved on 2014-05-23.
- ^ "Scientists uncover last steps for benzoic acid creation in plants". Purdue Agriculture News.
- ^ Neumüller O-A (1988). Römpps Chemie-Lexikon (6 ed.). Stuttgart: Frankh'sche Verlagshandlung. ISBN 978-3-440-04516-9. OCLC 50969944.
- ^ Liebig J; Wöhler F (1832). "Untersuchungen über das Radikal der Benzoesäure". Annalen der Chemie. 3 (3): 249–282. doi:10.1002/jlac.18320030302. hdl:2027/hvd.hxdg3f.
- ^ Salkowski E (1875). Berl Klin Wochenschr. 12: 297–298.
{{cite journal}}: Missing or empty|title=(help) - ^ Wade, Leroy G. (2014). Organic Chemistry (Pearson new international ed.). Harlow: Pearson Education Limited. p. 985. ISBN 978-1-292-02165-2.
- ^ D. D. Perrin; W. L. F. Armarego (1988). Purification of Laboratory Chemicals (3rd ed.). Pergamon Press. pp. 94. ISBN 978-0-08-034715-8.
- ^ Donald L. Pavia (2004). Introduction to Organic Laboratory Techniques: A Small Scale Approach. Thomson Brooks/Cole. pp. 312–314. ISBN 978-0-534-40833-6.
- ^ Shirley, D. A. (1954). "The Synthesis of Ketones from Acid Halides and Organometallic Compounds of Magnesium, Zinc, and Cadmium". Org. React. 8: 28–58.
- ^ Huryn, D. M. (1991). "Carbanions of Alkali and Alkaline Earth Cations: (ii) Selectivity of Carbonyl Addition Reactions". In Trost, B. M.; Fleming, I. (eds.). Comprehensive Organic Synthesis, Volume 1: Additions to C—X π-Bonds, Part 1. Elsevier Science. pp. 49–75. doi:10.1016/B978-0-08-052349-1.00002-0. ISBN 978-0-08-052349-1.
- ^ "The Grignard Reaction. Preparation of Benzoic Acid" (PDF). Portland Community College. Archived from the original (PDF) on 26 February 2015. Retrieved 12 March 2015.>
- ^ "Experiment 9: Synthesis of Benzoic Acid via Carbonylation of a Grignard Reagent" (PDF). University of Wisconsin-Madison. Archived from the original (PDF) on 23 September 2015. Retrieved 12 March 2015.
- ^ "Experiment 3: Preparation of Benzoic Acid" (PDF). Towson University. Archived from the original (PDF) on 13 April 2015. Retrieved 12 March 2015.>
- ^ Santonastaso, Marco; Freakley, Simon J.; Miedziak, Peter J.; Brett, Gemma L.; Edwards, Jennifer K.; Hutchings, Graham J. (21 November 2014). "Oxidation of Benzyl Alcohol using in Situ Generated Hydrogen Peroxide". Organic Process Research & Development. 18 (11): 1455–1460. doi:10.1021/op500195e. ISSN 1083-6160.
- ^ a b c d Maki, Takao; Takeda, Kazuo (2000). "Benzoic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_555. ISBN 978-3527306732..
- ^ A D Warth (1 December 1991). "Mechanism of action of benzoic acid on Zygosaccharomyces bailii: effects on glycolytic metabolite levels, energy production, and intracellular pH". Appl Environ Microbiol. 57 (12): 3410–4. Bibcode:1991ApEnM..57.3410W. doi:10.1128/AEM.57.12.3410-3414.1991. PMC 183988. PMID 1785916.
- ^ Pastrorova I, de Koster CG, Boom JJ (1997). "Analytic Study of Free and Ester Bound Benzoic and Cinnamic Acids of Gum Benzoin Resins by GC-MS HPLC-frit FAB-MS". Phytochem Anal. 8 (2): 63–73. doi:10.1002/(SICI)1099-1565(199703)8:2<63::AID-PCA337>3.0.CO;2-Y.
- ^ GSFA Online Food Additive Group Details: Benzoates (2006) Archived 26 September 2007 at the Wayback Machine
- ^ EUROPEAN PARLIAMENT AND COUNCIL DIRECTIVE No 95/2/EC of 20 February 1995 on food additives other than colours and sweeteners (Consleg-versions do not contain the latest changes in a law) Archived 19 April 2003 at the Wayback Machine
- ^ "Indications of the possible formation of benzene from benzoic acid in foods, BfR Expert Opinion No. 013/2006" (PDF). German Federal Institute for Risk Assessment. 1 December 2005. Archived (PDF) from the original on 26 April 2006. Retrieved 30 March 2022.
- ^ "Whitfield Ointment". Archived from the original on 9 October 2007. Retrieved 15 October 2007.
- ^ Charles Owens Wilson; Ole Gisvold; John H. Block (2004). Wilson and Gisvold's Textbook of Organic Medicinal and Pharmaceutical. Lippincott Williams & Wilkins. pp. 234. ISBN 978-0-7817-3481-3.
- ^ Lillard, Benjamin (1919). "Troches of Benzoic Acid". Practical Druggist and Pharmaceutical Review of Reviews.
- ^ Experiment 2: Using Bomb Calorimetry to Determine the Resonance Energy of Benzene Archived 9 March 2012 at the Wayback Machine
- ^ a b c "Concise International Chemical Assessment Document 26: BENZOIC ACID AND SODIUM BENZOATE".
- ^ Tomokuni K, Ogata M (1972). "Direct Colorimetric Determination of Hippuric Acid in Urine". Clin Chem. 18 (4): 349–351. doi:10.1093/clinchem/18.4.349. PMID 5012256.
- ^ Vogt, T. (2010). "Phenylpropanoid Biosynthesis". Molecular Plant. 3: 2–20. doi:10.1093/mp/ssp106. PMID 20035037.
- ^ Juteau, Pierre; Valérie Côté; Marie-France Duckett; Réjean Beaudet; François Lépine; Richard Villemur; Jean-Guy Bisaillon (January 2005). "Cryptanaerobacter phenolicus gen. nov., sp. nov., an anaerobe that transforms phenol into benzoate via 4-hydroxybenzoate". International Journal of Systematic and Evolutionary Microbiology. 55 (1): 245–250. doi:10.1099/ijs.0.02914-0. PMID 15653882.
- ^ Brewster, R. Q.; Williams, B.; Phillips, R. (1955). "3,5-Dinitrobenzoic Acid". Organic Syntheses; Collected Volumes, vol. 3, p. 337.
- ^ Cosmetic Ingredient Review Expert Panel Bindu Nair (2001). "Final Report on the Safety Assessment of Benzyl Alcohol, Benzoic Acid, and Sodium Benzoate". Int J Tox. 20 (Suppl. 3): 23–50. doi:10.1080/10915810152630729. PMID 11766131. S2CID 13639993.
- ^ "butyrate-CoA ligase". BRENDA. Technische Universität Braunschweig. Retrieved 7 May 2014. Substrate/Product
- ^ "glycine N-acyltransferase". BRENDA. Technische Universität Braunschweig. Retrieved 7 May 2014. Substrate/Product
- ^ Krebs HA, Wiggins D, Stubbs M (1983). "Studies on the mechanism of the antifungal action of benzoate". Biochem J. 214 (3): 657–663. doi:10.1042/bj2140657. PMC 1152300. PMID 6226283.
- ^ Bedford PG, Clarke EG (1972). "Experimental benzoic acid poisoning in the cat". Vet Rec. 90 (3): 53–58. doi:10.1136/vr.90.3.53 (inactive 1 November 2024). PMID 4672555. S2CID 2553612.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Chen, Jian; Y.L. Kao (18 January 2010). "Nearly 30% dried, pickled foods fail safety inspections". The China Post.