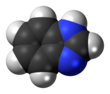
Benzimidazole

| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1H-1,3-Benzimidazole | |||
| Other names 1H-Benzo[d]imidazole | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 109682 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.075 | ||
| EC Number |
| ||
| 3106 | |||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C7H6N2 | |||
| Molar mass | 118.139 g·mol−1 | ||
| Melting point | 170 to 172 °C (338 to 342 °F; 443 to 445 K) | ||
| Acidity (pKa) | 12.8 (for benzimidazole) and 5.6 (for the conjugate acid)[1] | ||
| Hazards | |||
| GHS labelling: | |||

| |||
| Warning | |||
| H302, H315, H319, H335 | |||
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |||
| Safety data sheet (SDS) | External MSDS | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Benzimidazole is a heterocyclic aromatic organic compound. This bicyclic compound may be viewed as fused rings of the aromatic compounds benzene and imidazole. It is a white solid that appears in form of tabular crystals.[2]
Preparation
Benzimidazole was discovered during research on vitamin B12. The benzimidazole nucleus was found to be a stable platform on which drugs could be developed.[3] Benzimidazole is produced by condensation of o-phenylenediamine with formic acid,[4] or the equivalent trimethyl orthoformate:
- C6H4(NH2)2 + HC(OCH3)3 → C6H4N(NH)CH + 3 CH3OH
2-Substituted derivatives are obtained when the condensation is conducted with aldehydes in place of formic acid, followed by oxidation.[5]
Reactions
Benzimidazole is a base:
- C6H4N(NH)CH + H+ → [C6H4(NH)2CH]+
It can also be deprotonated with stronger bases:
- C6H4N(NH)CH + LiH → Li [C6H4N2CH] + H2
The imine can be alkylated and also serves as a ligand in coordination chemistry. The most prominent benzimidazole complex features N-ribosyl-dimethylbenzimidazole, as found in vitamin B12.[6]
N,N'-Dialkylbenzimidazolium salts are precursors to certain N-heterocyclic carbenes.[7][8]
Applications
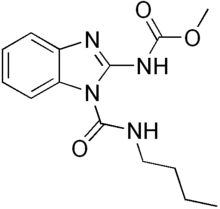
Benzimidazole derivatives are among the most frequently used ring systems for small molecule drugs listed by the United States Food and Drug Administration.[9] Many pharmaceutical agents belong to the benzimidazole class of compounds. For example:
- Angiotensin II receptor blockers such as azilsartan, candesartan, and telmisartan.
- Anthelmintic agents such as albendazole, ciclobendazole, fenbendazole, flubendazole, mebendazole, oxfendazole, oxibendazole, triclabendazole, and thiabendazole. These drugs work by binding tubulin, a vital part of the cytoskeleton and mitotic spindle. Benzimidazoles are selectively toxic towards parasitic nematodes, selectively binding and depolymerising their tubulins.[10]
- Antihistamines such as astemizole, bilastine, clemizole, emedastine, mizolastine, and oxatomide.
- Benzimidazole fungicides such as benomyl, carbendazim, fuberidazole, and thiabendazole. These drugs selectively bind to and depolymerise fungal tubulin.[10]
- Benzimidazole opioids such as bezitramide, brorphine, clonitazene, etodesnitazene, etonitazene, etonitazepipne, etonitazepyne, isotonitazene, metodesnitazene, and metonitazene.
- Proton-pump inhibitors such as dexlansoprazole, esomeprazole, ilaprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole, and tenatoprazole.
- Typical antipsychotics such as benperidol, clopimozide, droperidol, neflumozide, and oxiperomide, and pimozide.
- Other notable pharmaceutical agents which contain a benzimidazole group include abemaciclib, bendamustine, dabigatran, daridorexant, and glasdegib.
In printed circuit board manufacturing, benzimidazole can be used as an organic solderability preservative.[citation needed]
Several dyes are derived from benzimidazoles.[11]
See also
- Benzimidazoline
- Polybenzimidazole, a high performance fiber
References
- ^ Walba, Harold; Isensee, Robert W. (1961). "Acidity Constants of Some Arylimidazoles and Their Cations". The Journal of Organic Chemistry. 26 (8): 2789–2791. doi:10.1021/jo01066a039.
- ^ "Benzimidazole | CAMEO Chemicals | NOAA". cameochemicals.noaa.gov. Retrieved 2023-01-11.
- ^ Bennet-Jenkins, E.; Bryant, C. (1996). "Novel sources of anthelmintics". International Journal for Parasitology. 26 (8–9): 937–947. doi:10.1016/s0020-7519(96)80068-3. ISSN 0020-7519. PMID 8923141.
- ^ E. C. Wagner, W. H. Millett (1939). "Benzimidazole". Organic Syntheses. 19: 12. doi:10.15227/orgsyn.019.0012.
- ^ Smiley, Robert A. (2000), "Phenylene- and Toluenediamines", Ullmann's Encyclopedia of Industrial Chemistry, doi:10.1002/14356007.a19_405, ISBN 978-3-527-30385-4
- ^ H. A. Barker; R. D. Smyth; H. Weissbach; J. I. Toohey; J. N. Ladd & B. E. Volcani (February 1, 1960). "Isolation and Properties of Crystalline Cobamide Coenzymes Containing Benzimidazole or 5,6-Dimethylbenzimidazole". Journal of Biological Chemistry. 235 (2): 480–488. doi:10.1016/S0021-9258(18)69550-X. PMID 13796809.
- ^ R. Jackstell; A. Frisch; M. Beller; D. Rottger; M. Malaun; B. Bildstein (2002). "Efficient telomerization of 1,3-butadiene with alcohols in the presence of in situ generated palladium(0)carbene complexes". Journal of Molecular Catalysis A: Chemical. 185 (1–2): 105–112. doi:10.1016/S1381-1169(02)00068-7.
- ^ H. V. Huynh; J. H. H. Ho; T. C. Neo; L. L. Koh (2005). "Solvent-controlled selective synthesis of a trans-configured benzimidazoline-2-ylidene palladium(II) complex and investigations of its Heck-type catalytic activity". Journal of Organometallic Chemistry. 690 (16): 3854–3860. doi:10.1016/j.jorganchem.2005.04.053.
- ^ Taylor, R. D.; MacCoss, M.; Lawson, A. D. G. J Med Chem 2014, 57, 5845.>
- ^ a b Wang, C. C. (January 1984). "Parasite enzymes as potential targets for antiparasitic chemotherapy". Journal of Medicinal Chemistry. 27 (1): 1–9. doi:10.1021/jm00367a001. ISSN 0022-2623. PMID 6317859.
- ^ Berneth, Horst (2008), "Methine Dyes and Pigments", Ullmann's Encyclopedia of Industrial Chemistry, doi:10.1002/14356007.a16_487.pub2, ISBN 978-3-527-30385-4
Further reading
- Grimmett, M. R. (1997). Imidazole and benzimidazole synthesis. Boston: Academic Press. ISBN 0-12-303190-7.