Allenestrol
 | |
| Clinical data | |
|---|---|
| Other names | Allenestril; Allenoestril; α,α-Dimethyl-β-ethylallenolic acid; Dimethylethylallenolic acid; Methallenestrilphenol; Methallenestrolphenol |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
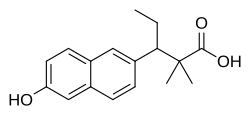
| Formula | C17H20O3 |
| Molar mass | 272.344 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Allenestrol, or allenoestrol, also known as α,α-dimethyl-β-ethylallenolic acid or as methallenestrilphenol, is a synthetic, nonsteroidal estrogen and a derivative of allenolic acid that was never marketed.[1][2][3] A methyl ether of allenestrol, methallenestril (methallenestrol), is also an estrogen, but, in contrast to allenestrol, has been marketed.[4]
See also
References
- ^ Negwer M, Scharnow HG (4 October 2001). Organic-chemical drugs and their synonyms: (an international survey). Wiley-VCH. ISBN 978-3-527-30247-5.
C17H2003. 15372-37-9. 2,2-Dimethyl-3-(6-hydroxy-2-naphthyl)valeric acid = α,α-Dimethyl-β-ethylallenolic acid = (±)-β-Ethyl-6-hydroxy-α,α-dimethyl-2-naphthalenepropanoic acid (•) S: Allenestrol, Allenoestrol. U: Estrogen.
- ^ Wermuth CG (11 June 2003). The Practice of Medicinal Chemistry. Academic Press. pp. 216–. ISBN 978-0-08-049777-8.
- ^ Vermuth CG, Aldous D, Raboisson P, Rognan D (1 July 2015). The Practice of Medicinal Chemistry. Elsevier Science. pp. 245–. ISBN 978-0-12-417213-5.
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 781–. ISBN 978-1-4757-2085-3.
