4-Aminophenol
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 4-Aminophenol[1] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| 385836 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.198 | ||
| EC Number |
| ||
| 2926 | |||
| KEGG | |||
| MeSH | Aminophenols | ||
PubChem CID |
|||
| UNII | |||
| UN number | 2512 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H7NO | |||
| Molar mass | 109.128 g·mol−1 | ||
| Appearance | Colorless to reddish-yellow crystals | ||
| Density | 1.13 g/cm3 | ||
| Melting point | 187.5 °C (369.5 °F; 460.6 K) | ||
| Boiling point | 284 °C (543 °F; 557 K) | ||
| 1.5 g/100 mL | |||
| Solubility |
| ||
| log P | 0.04 | ||
| Acidity (pKa) |
| ||
| Structure | |||
| orthorhombic | |||
| Thermochemistry | |||
Std enthalpy of formation (ΔfH⦵298) |
-190.6 kJ/mol | ||
| Hazards | |||
| GHS labelling: | |||
  
| |||
| Warning | |||
| H302, H332, H341, H410 | |||
| P201, P202, P261, P264, P270, P271, P273, P281, P301+P312, P304+P312, P304+P340, P308+P313, P312, P330, P391, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 195 °C (383 °F; 468 K) (cc) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
671 mg/kg | ||
| Related compounds | |||
Related aminophenols |
2-Aminophenol 3-Aminophenol | ||
Related compounds |
Aniline Phenol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
4-Aminophenol (or para-aminophenol or p-aminophenol) is an organic compound with the formula H2NC6H4OH. Typically available as a white powder,[3] it is commonly used as a developer for black-and-white film, marketed under the name Rodinal.
Reflecting its slightly hydrophilic character, the white powder is moderately soluble in alcohols and can be recrystallized from hot water. In the presence of a base, it oxidizes readily. The methylated derivatives N-methylaminophenol and N,N-dimethylaminophenol are of commercial value.
The compound is one of three isomeric aminophenols, the other two being 2-aminophenol and 3-aminophenol.
Preparation
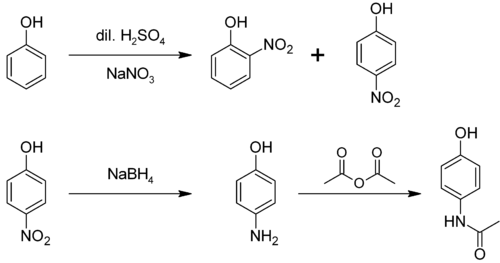
From phenol
It is produced from phenol by nitration followed by reduction with iron. Alternatively, the partial hydrogenation of nitrobenzene affords phenylhydroxylamine, which rearranges primarily to 4-aminophenol (Bamberger rearrangement).[4]
- C6H5NO2 + 2 H2 → C6H5NHOH + H2O
- C6H5NHOH → HOC6H4NH2
From nitrobenzene
It can be produced from nitrobenzene by electrolytic conversion to phenylhydroxylamine, which spontaneously rearranges to 4-aminophenol.[5]
From 4-nitrophenol
4-nitrophenol can be reduced through a variety of methods, to yield 4-aminophenol. One method involves hydrogenation over a Raney Nickel catalyst. A second method involves selective reduction of the nitro group by Tin(II) Chloride in anhydrous ethanol or ethyl ethanoate. [6][7]
Uses
4-Aminophenol is a building block used in organic chemistry. Prominently, it is the final intermediate in the industrial synthesis of paracetamol. Treating 4-aminophenol with acetic anhydride gives paracetamol:[8][9][10]
It is a precursor to amodiaquine, mesalazine, AM404, parapropamol, B-86810 & B-87836 (c.f. WO 2001042204).
4-Aminophenol converts readily to the diazonium salt.[11]
References
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 690. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. pp. 5–89. ISBN 978-1498754286.
- ^ CRC Handbook of Chemistry and Physics 65th Ed.
- ^ Mitchell, S.C. & Waring, R.H. "Aminophenols." In Ullmann’s Encyclopedia of Industrial Chemistry; 2002 Wiley-VCH, doi:10.1002/14356007.a02_099
- ^ Polat, K.; Aksu, M.L.; Pekel, A.T. (2002), "Electroreduction of nitrobenzene to p-aminophenol using voltammetric and semipilot scale preparative electrolysis techniques", Journal of Applied Electrochemistry, 32 (2), Kluwer Academic Publishers: 217–223, doi:10.1023/A:1014725116051, S2CID 54499902
- ^ US2998450A, Godfrey, Wilbert & De, Angelis John, "Process of preparing nu-acetyl-p-amino phenol", issued 1961-08-29
- ^ Bellamy, F. D.; Ou, K. (1984-01-01). "Selective reduction of aromatic nitro compounds with stannous chloride in non acidic and non aqueous medium". Tetrahedron Letters. 25 (8): 839–842. doi:10.1016/S0040-4039(01)80041-1. ISSN 0040-4039.
- ^ Ellis, Frank (2002). Paracetamol: a curriculum resource. Cambridge: Royal Society of Chemistry. ISBN 0-85404-375-6.
- ^ Anthony S. Travis (2007). "Manufacture and uses of the anilines: A vast array of processes and products". In Zvi Rappoport (ed.). The chemistry of Anilines Part 1. Wiley. p. 764. ISBN 978-0-470-87171-3.
- ^ Elmar Friderichs; Thomas Christoph; Helmut Buschmann. "Analgesics and Antipyretics". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_269.pub2. ISBN 978-3527306732.
- ^ F. B. Dains, Floyd Eberly (1935). "p-Iodophenol". Organic Syntheses. 15: 39. doi:10.15227/orgsyn.015.0039.