Zethrene

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name Dibenzo[de,mn]tetracene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C24H14 | |
| Molar mass | 302.376 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Zethrene (dibenzo[de,mn]naphthacene) is a polycyclic aromatic hydrocarbon consisting of two phenalene units fused together. According to Clar's rule, the two exterior naphthalene units are truly aromatic and the two central double bonds are not aromatic at all. For this reason the compound is of some interest to academic research. Zethrene has a deep-red color and it is light sensitive - complete decomposition under a sunlight lamp occurs within 12 hours. The melting point is 262 °C.
Synthesis
The compound was originally synthesized by Erich Clar in 1955[1] from acenaphthene in one method and from chrysene in another. Mitchell and Sondheimer prepared the compound from a benzannulated [10]annulene.[2][3]
 |
| Zethrene synthesis (Sondheimer 1968) |
|---|
A sulfur extrusion method was reported by Kemp, Storie, and Tulloch.[4] Wu et al.[5] reported the synthesis of the compound in a coupling reaction / dimerization with in-situ desilylation.
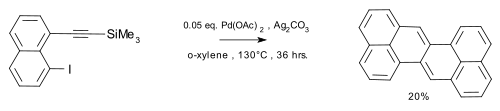 |
| Zethrene synthesis (Wu 2010) |
|---|
A Heck variation was reported in 2013.[6]
Derivatives are also known.[7][8]
Structure
X-ray crystallography indicates that zethrene is a planar molecule.[5] The bond lengths in the central part of the molecule are consistent with distinct single and double bonds rather than aromatic components.
References
- ^ Clar, Erich; Lang, Karl Friedrich; Schulz-Kiesow, Hans (1955). "Aromatische Kohlenwasserstoffe, LXX. Mitteil.1): Zethren (1.12; 6.7-Dibenztetracen)". Chemische Berichte. 88 (10): 1520. doi:10.1002/cber.19550881008.
- ^ Mitchell, Reginald Harry; Sondheimer, Franz (1968). "A dinaphth[10]annulene". Journal of the American Chemical Society. 90 (2): 530. doi:10.1021/ja01004a080.
- ^ Mitchell, R.H.; Sondheimer, F. (1970). "The attempted synthesis of a dinaphth-1,6-bisdehydro[10]annulene". Tetrahedron. 26 (9): 2141. doi:10.1016/S0040-4020(01)92792-9.
- ^ Kemp, William; Storie, Iain T.; Tulloch, Charles D. (1980). "Synthesis of potentially basic hydrocarbons by sulphur extrusion and/or bis-Wittig reactions. Two syntheses of benz[5,6]indeno[2,1-a]phenalene and a new synthesis of dibenzo[de,mn]naphthacene (zethrene)". Journal of the Chemical Society, Perkin Transactions 1: 2812. doi:10.1039/P19800002812.
- ^ a b Wu, Tsun-Cheng; Chen, Chia-Hua; Hibi, Daijiro; Shimizu, Akihiro; Tobe, Yoshito; Wu, Yao-Ting (2010). "Synthesis, Structure, and Photophysical Properties of Dibenzo[de,mn]naphthacenes". Angewandte Chemie International Edition. 49 (39): 7059–7062. doi:10.1002/anie.201001929. PMID 20715235.
- ^ Shan, Liang; Liang, Zhixiong; Xu, Xiaomin; Tang, Qin; Miao, Qian (2013). "Revisiting zethrene: Synthesis, reactivity and semiconductor properties". Chemical Science. 4 (8): 3294. doi:10.1039/C3SC51158H.
- ^ Umeda, Rui; Hibi, Daijiro; Miki, Koji; Tobe, Yoshito (2009). "Tetradehydrodinaphtho[10]annulene: A Hitherto Unknown Dehydroannulene and a Viable Precursor to Stable Zethrene Derivatives". Organic Letters. 11 (18): 4104–4106. doi:10.1021/ol9015942. PMID 19673535.
- ^ Sun, Zhe; Huang, Kuo-Wei; Wu, Jishan (2010). "Soluble and Stable Zethrenebis(dicarboximide) and Its Quinone". Organic Letters. 12 (20): 4690–4693. doi:10.1021/ol102088j. PMID 20863074.
