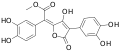
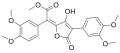
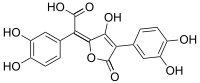
Variegatic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name (E)-(3,4-Dihydroxyphenyl)[4-(3,4-dihydroxyphenyl)-3-hydroxy-5-oxofuran-2(5H)-ylidene]acetic acid | |
| Other names 3,3′,4,4′-Tetrahydroxy pulvinic acid | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H12O9 | |
| Molar mass | 372.285 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Variegatic acid (3,3',4,4'-tetrahydroxypulvinic acid) is an orange pigment found in some mushrooms. It is responsible for the bluing reaction seen in many bolete mushrooms when they are injured. When mushroom tissue containing variegatic acid is exposed to air, the chemical is enzymatically oxidized to blue quinone methide anions, specifically chinonmethid anions.[1] It is derived from xerocomic acid, which is preceded by atromentic acid and atromentin, and its genetic basis is unknown. In its oxidized form (due to the production of a second lactone ring) is variegatorubin, similar to xerocomorubin.
It was first isolated from Suillus variegatus.[2] It has strong antioxidant properties,[3][4] and a nonspecific inhibitory effect on cytochrome P450 enzymes.[5] A total synthesis was reported in 2001 that uses a Suzuki cross coupling reaction.[6] It was found antibiotically inactive against an array of bacteria and fungi using the disk diffusion assay at 50 μg.[7] However, at similar concentrations it was found to inhibit swarming and (probably consequently) biofilm formation of Bacillus subtilis. In vitro data supports that this pigment is an Fe3+-reducant in Fenton chemistry during the initial attack of dead plant matter as part of the brown-rot saprobic lifestyle.[8]
Derivatives
Variegatic acid methyl ester, 3-O-methylvariegatic acid methyl ester, and 3,3',4,4'-tetra-O-methylvariegatic acid methyl ester are red-orange pigments found in Boletales.[9][10]
- Variegatic acid methyl ester
- 3-O-Methylvariegatic acid methyl ester
- 3,3',4,4'-Tetra-O-methyl variegatic acid methyl ester
See also
References
- ^ Velíšek J, Cejpek K (2011). "Pigments of higher fungi: A review" (PDF). Czech Journal of Food Sciences. 29 (2): 87–102. doi:10.17221/524/2010-CJFS.
- ^ Edwards RL, Elsworthy GC (1967). "Variegatic acid, a new tetronic acid responsible for the blueing reaction in the fungus Suillus (Boletus) variegatus (Swartz ex Fr.)". Chemical Communications (8): 373b–374. doi:10.1039/C1967000373B.
- ^ Kasuga A, Aoyagi Y, Sugahara T (1995). "Antioxidant activity of fungus Suillus bovinus (L: Fr.) O. Kuntze". Journal of Food Science. 60 (5): 1113–85. doi:10.1111/j.1365-2621.1995.tb06304.x.
- ^ Vidovic SS, Mujic IO, Zekovic ZP, Lepojevic ZD, Tumbas VT, Mujic AI (2010). "Antioxidant properties of selected Boletus mushrooms". Food Biophysics. 5 (1): 49–58. doi:10.1007/s11483-009-9143-6. S2CID 84061662.
- ^ Huang YT, Onose J, Abe N, Yoshikawa K (2009). "In vitro inhibitory effects of pulvinic acid derivatives isolated from Chinese edible mushrooms, Boletus calopus and Suillus bovinus, on cytochrome P450 activity". Bioscience, Biotechnology, and Biochemistry. 73 (4): 855–60. doi:10.1271/bbb.80759. PMID 19352038. S2CID 39654350.

- ^ Ahmed Z, Langer P (2005). "Synthesis of natural pulvinic acids based on a '[3+2] cyclization-Suzuki cross-coupling' strategy". Tetrahedron. 61 (8): 2055–63. doi:10.1016/j.tet.2004.12.048.
- ^ Tauber, J. P., Schroeckh, V., Shelest, E., Brakhage, A. A. and Hoffmeister, D. (2016), Bacteria induce pigment formation in the basidiomycete Serpula lacrymans. Environ Microbiol, 18: 5218–5227. doi:10.1111/1462-2920.13558
- ^ Eastwood et al. (2011) The Plant Cell Wall- Decomposing Machinery Underlies the Functional Diversity of Forest Fungi. Science.
- ^ Gruber, Gertraud (2002). "Isolierung und Strukturaufklärung von chemotaxonomisch relevanten Sekundärmetaboliten aus höheren Pilzen, insbesondere aus der Ordnung der Boletales" (PDF). edoc.ub.uni-muenchen.de (in German). Retrieved 2023-08-10.
- ^ Gill, M., and Steglich, W. (1987) Pigments of fungi (Macromycetes). Prog Chem Org Nat Prod 51: 1–317.