Tebufenozide
| |
| Names | |
|---|---|
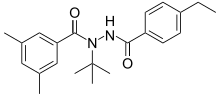
| Preferred IUPAC name N-tert-Butyl-N′-(4-ethylbenzoyl)-3,5-dimethylbenzohydrazide | |
| Other names Mimic, RH-75992, HOE-105540, Confirm 2F, Confirm 70 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.101.212 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C22H28N2O2 | |
| Molar mass | 352.478 g·mol−1 |
| Melting point | 191 to 191.5 °C (375.8 to 376.7 °F; 464.1 to 464.6 K)[1] |
| 0.83 mg/L[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Tebufenozide is an insecticide that acts as a molting hormone. It is an agonist of the ecdysone receptor that causes premature molting in larvae. It is primarily used against caterpillar pests.[2] It belongs to the class of diacylhydrazines.[3]
Because it has high selectivity for the targeted pests and low toxicity otherwise, the company that discovered tebufenozide, Rohm and Haas, was given a Presidential Green Chemistry Award for its development.[2]
Its environmental half-life varies according to where it is released and under what conditions, but can be said to be on the order of months.[4]
It is used as an insect growth regulator, to control leaf-eating insects that cause damage or death in trees. Tebufenozide is the active ingredient in" Bayer's MIMIC formulation, which controls forest defoliator pests such as gypsy moths, tent caterpillars, budworms, tussock moths and cabbage looper. These are all pests of the order Lepidoptera.[5]
It has been used against the sugarcane borer, although the population grows immunity.[6]
In California, the substance was used chiefly for crops of head lettuce, celery, raspberries, cauliflower, and tomatoes for processing.
A 1994 study conducted by the Canadian Forest Service in laboratory conditions concluded that the substance was very stable in acidic and neutral buffers at 20 °C, hydrolytic degradation was dependent on pH and temperature, sunlight photodegradation was observed at a slower rate than ultraviolet photodegradation, and that microbial metabolism and photolysis are the two main degradative routes for tebufenozide in natural aquatic systems.[7]
The final degradation products of tebufenozide are various alcohols, carboxylic acids and ketones of low toxicity.[8]
References
- ^ a b Tebufenozide, Food and Agriculture Organization of the United Nations
- ^ a b Carlson, Glenn R. (2000). "Tebufenozide: A Novel Caterpillar Control Agent with Unusually High Target Selectivity". Green Chemical Syntheses and Processes. ACS Symposium Series. Vol. 767. pp. 8–17. doi:10.1021/bk-2000-0767.ch002. ISBN 978-0-8412-3678-3.
- ^ Dhadialla, Tarlochan S.; Carlson, Glenn R.; Le, Dat P. (1998). "New Insecticides with Ecdysteroidal and Juvenile Hormone Activity". Annual Review of Entomology. 43: 545–569. doi:10.1146/annurev.ento.43.1.545. PMID 9444757.
- ^ pubchem: "Tebufenozide"
- ^ "Controlling forest insects with Mimic®", 2017-07-26
- ^ Reay-Jones, F.P.F.; Akbar, W.; McAllister, C. D.; Reagan, T. E.; Ottea, J. A. (2005). "Reduced Susceptibility to Tebufenozide in Populations of the Sugarcane Borer (Lepidoptera: Crambidae) in Louisiana". Journal of Economic Entomology. 98 (3): 955–960. doi:10.1603/0022-0493-98.3.955. PMID 16022328.
- ^ Sundaram, K. M. S. (1994). "Degradation kinetics of tebufenozide in model aquatic systems under controlled laboratory conditions". Journal of Environmental Science and Health, Part B. 29 (6): 1081–1104. Bibcode:1994JESHB..29.1081S. doi:10.1080/03601239409372917.
- ^ Roberts TR et al, "Metabolic Pathways of Agrochemicals: Part 2: Insecticides and Fungicides", p820 (Royal Society of Chemistry, 2007)
