Suxibuzone
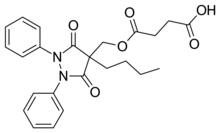 Suxibuzone molecule | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.044.056 |
| Chemical and physical data | |
| Formula | C24H26N2O6 |
| Molar mass | 438.480 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Suxibuzone is an analgesic used for joint and muscular pain. It is a prodrug of the non-steroidal anti-inflammatory drug (NSAID) phenylbutazone,[1] and is commonly used in horses.[2]
Synthesis
Suxibuzone is synthesized by the following method:[3] Patent:[4] (Precursor:[5])

Phenylbutazone [50-33-9] (1) is hydroxymethylated with formaldehyde giving ~86% 4-butyl-4-(hydroxymethyl)-1,2-diphenylpyrazolidine-3,5-dione [23111-33-3] (2). This is then esterified with succinic anhydride. [108-30-5] (3) to give (4).
References
- ^ Yasuda Y, Shindo T, Mitani N, Ishida N, Oono F, Kageyama T (1982). "Comparison of the absorption, excretion, and metabolism of suxibuzone and phenylbutazone in humans". Journal of Pharmaceutical Sciences. 71 (5): 565–72. doi:10.1002/jps.2600710521. PMID 7097505.
- ^ Sabaté D, Homedes J, Salichs M, Sust M, Monreal L (2009). "Multicentre, controlled, randomised and blinded field study comparing efficacy of suxibuzone and phenylbutazone in lame horses". Equine Veterinary Journal. 41 (7): 700–5. doi:10.2746/042516409X464807. PMID 19927590.
- ^ Esteve, J. et al, Quim. Ind. (Madrid), 1971,17, 107.
- ^ Esteve Dr Antonio, DE1936747A1 (1970 to Laboratorios Del Dr. Esteve S.A., Barcelona (Spanien)).
- ^ Dhareshwar, Sundeep S.; Stella, Valentino J. (2010). "A Novel Prodrug Strategy for β-Dicarbonyl Carbon Acids: Syntheses and Evaluation of the Physicochemical Characteristics of C-Phosphoryloxymethyl (POM) and Phosphoryloxymethyloxymethyl (POMOM) Prodrug Derivatives". Journal of Pharmaceutical Sciences. 99 (6): 2711–2723. doi:10.1002/jps.22021.
