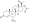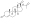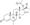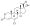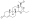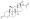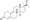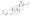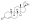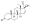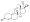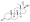Progestogen (medication)
| Progestogen (medication) | |
|---|---|
| Drug class | |
 Progesterone, the natural progestogen in the body and one of the most widely used progestogen medications | |
| Class identifiers | |
| Synonyms | Progestagen, gestagen, gestogen; progestin (synthetic progestogen); progesterone receptor agonist |
| Use | Hormonal birth control, hormone therapy, gynecological disorders, fertility medicine and pregnancy support, sex-hormone suppression, others |
| ATC code | G03 |
| Biological target | Progesterone receptors (PR-A, PR-B, PR-C); membrane progesterone receptors (mPRα, mPRβ, mPRγ, mPRδ, mPRε); progesterone receptor membrane components (PGRMC1, PGRMC2) |
| Chemical class | Steroids (pregnanes, norpregnanes, retropregnanes, androstanes, estranes) |
| Clinical data | |
| Drugs.com | Drug Classes |
| External links | |
| MeSH | D011372 |
| Legal status | |
| In Wikidata | |
A progestogen, also referred to as a progestagen, gestagen, or gestogen, is a type of medication which produces effects similar to those of the natural female sex hormone progesterone in the body.[1] A progestin is a synthetic progestogen.[1] Progestogens are used most commonly in hormonal birth control and menopausal hormone therapy.[1] They can also be used in the treatment of gynecological conditions, to support fertility and pregnancy, to lower sex hormone levels for various purposes, and for other indications.[1] Progestogens are used alone or in combination with estrogens.[1] They are available in a wide variety of formulations and for use by many different routes of administration.[1] Examples of progestogens include natural or bioidentical progesterone as well as progestins such as medroxyprogesterone acetate and norethisterone.[1]
Side effects of progestogens include menstrual irregularities, headaches, nausea, breast tenderness, mood changes, acne, increased hair growth, and changes in liver protein production among others.[1][2] Other side effects of progestogens may include an increased risk of breast cancer, cardiovascular disease, and blood clots.[2] At high doses, progestogens can cause low sex hormone levels and associated side effects like sexual dysfunction and an increased risk of bone fractures.[3]
Progestogens are agonists of the progesterone receptors (PRs) and produce progestogenic, or progestational, effects.[1] They have important effects in the female reproductive system (uterus, cervix, and vagina), the breasts, and the brain.[1] In addition, many progestogens also have other hormonal activities, such as androgenic, antiandrogenic, estrogenic, glucocorticoid, or antimineralocorticoid activity.[1] They also have antigonadotropic effects and at high doses can strongly suppress sex hormone production.[1] Progestogens mediate their contraceptive effects both by inhibiting ovulation and by thickening cervical mucus, thereby preventing fertilization.[4][5] They have functional antiestrogenic effects in certain tissues like the endometrium, and this underlies their use in menopausal hormone therapy.[1]
Progesterone was first introduced for medical use in 1934 and the first progestin, ethisterone, was introduced for medical use in 1939.[6][7][8] More potent progestins, such as norethisterone, were developed and started to be used in birth control in the 1950s.[6] Around 60 progestins have been marketed for clinical use in humans or use in veterinary medicine.[9][10][11][12][13] These progestins can be grouped into different classes and generations.[1][14][15] Progestogens are available widely throughout the world and are used in all forms of hormonal birth control and in most menopausal hormone therapy regimens.[1][9][10][12][11]
Medical uses
Available forms
Progestogens are available in many different forms for use by many different routes of administration. These include oral tablets and capsules, oil and aqueous solutions and suspensions for intramuscular or subcutaneous injection, and various others (e.g., transdermal patches, vaginal rings, intrauterine devices, subcutaneous implants).
Dozens of different progestogens have been marketed for clinical and/or veterinary use.
Birth control
Progestogens are used in a variety of different forms of hormonal birth control for females, including combined estrogen and progestogen forms like combined oral contraceptive pills, combined contraceptive patches, combined contraceptive vaginal rings, and combined injectable contraceptives; and progestogen-only forms like progestogen-only contraceptive pills ("mini-pills"), progestogen-only emergency contraceptive pills ("day-after pills"), progestogen-only contraceptive implants, progestogen-only intrauterine devices, progestogen-only contraceptive vaginal rings, and progestogen-only injectable contraceptives.[16][17][18][19]
Progestogens mediate their contraceptive effects by multiple mechanisms, including prevention of ovulation via their antigonadotropic effects; thickening of cervical mucus, making the cervix largely impenetrable to sperm; preventing capacitation of sperm due to changes in cervical fluid, thereby making sperm unable to penetrate the ovum; and atrophic changes in the endometrium, making the endometrium unsuitable for implantation.[20][21][22][23] They may also decrease tubal motility and ciliary action.[23]
Hormone therapy
Menopause and hypogonadism
Progestogens are used in combination with estrogens in menopausal hormone therapy in women. They are also used in combination with estrogens in hormone therapy for hypogonadism and delayed puberty in girls and women. They are used mainly to prevent endometrial hyperplasia and increased risk of endometrial cancer from unopposed estrogen therapy.
Transgender hormone therapy
Progestogens are used as a component of hormone therapy for transgender women and transgender men. They are used in transgender women in combination with estrogens to help suppress and block testosterone. Progestogens might also have other beneficial effects in transgender women, but these are controversial and unsupported at present. Examples of progestogens used in hormone therapy for transgender women include cyproterone acetate, medroxyprogesterone acetate, and progesterone. Progestogens, such as medroxyprogesterone and lynestrenol, are used in transgender men to help suppress menses. Progestogens have also been used to delay puberty in transgender boys and girls.
Other uses
Certain progestogens, including megestrol acetate, medroxyprogesterone acetate, cyproterone acetate, and chlormadinone acetate, have been used at high doses to reduce hot flashes in men undergoing androgen deprivation therapy, for instance to treat prostate cancer.[24][25][26]
Gynecological disorders
Menstrual disorders
Progestogens are used to treat menstrual disorders such as secondary amenorrhea and dysfunctional uterine bleeding.[17][18] In a normal menstrual cycle, declining levels of progesterone trigger menstruation. Progestogens such as norethisterone acetate and medroxyprogesterone acetate may be used to artificially induce progesterone-associated breakthrough bleeding.[27]
The progestogen challenge test or progestogen withdrawal test is used to diagnose amenorrhea. Due to the availability of assays to measure estrogen levels, it is now rarely used.
Uterine disorders
Progestogens are used in the prevention and treatment of uterine disorders such as endometrial hyperplasia, endometriosis, uterine fibroids, and uterine hypoplasia.
Breast disorders
Progestogens are used to treat benign breast disorders.[28][29] They are associated not only with a reduction in breast pain, but also a decrease in breast cell proliferation, a decrease in breast gland size, and a disappearance of breast nodularity.[28][29][30] Progestogens that have been used for such purposes include topical progesterone, dydrogesterone, promegestone, lynestrenol, medroxyprogesterone acetate, dienogest, and medrogestone.[28][29][31][30]
Progestogens are used in the treatment of breast hypoplasia and lactation insufficiency. This is because they induce lobuloalveolar development of the breasts, which is required for lactation and breastfeeding.
Enlarged prostate
Progestogens have been used at high doses to treat benign prostatic hyperplasia (BPH). They act by suppressing gonadal testosterone production and hence circulating testosterone levels. Androgens like testosterone stimulate the growth of the prostate gland.
Hormone-sensitive cancers
Endometrial cancer
Progestogens were first found to be effective at high doses in the treatment of endometrial hyperplasia and endometrial cancer in 1959.[32][33][34] Subsequently, high-dose gestonorone caproate, hydroxyprogesterone caproate, medroxyprogesterone acetate, and megestrol acetate were approved for the treatment of endometrial cancer.[35][36][37]
Breast cancer
Progestogens, such as megestrol acetate and medroxyprogesterone acetate, are effective at high doses in the treatment of advanced postmenopausal breast cancer.[38][39] They have been extensively evaluated as a second-line therapy for this indication.[38] However, they produce various side effects, such as dyspnea, weight gain, vaginal bleeding, nausea, fluid retention, hypertension, thrombophlebitis, and thromboembolic complications.[38][39] In addition, megestrol acetate has been found to be significantly inferior to aromatase inhibitors in the treatment of breast cancer, and in relation to this, progestogens have been moved down in the sequential therapy of the disease.[38] Megestrol acetate is the only Food and Drug Administration-approved progestogen for breast cancer.[38] The mechanism of action of progestogens in the treatment of breast cancer is unknown, but may be related to their functional antiestrogenic and/or antigonadotropic effects.[38]
Prostate cancer
Certain progestogens, particularly those with antiandrogenic properties, have been used at high doses in the treatment of prostate cancer.[40][41] These include cyproterone acetate, chlormadinone acetate, and megestrol acetate.[40][41] Other progestogens such as medroxyprogesterone acetate, hydroxyprogesterone caproate, and gestonorone caproate have also been studied, but have inadequate effectiveness. They act by suppressing gonadal testosterone production and hence circulating testosterone levels. Androgens like testosterone stimulate the growth of prostate tumors.
Fertility and pregnancy
Progestogens are used in fertility medicine for women. For example, progesterone (or sometimes dydrogesterone or hydroxyprogesterone caproate) is used for luteal support in in-vitro fertilization protocols.[42]
Certain progestogens are used to support pregnancy, including progesterone, hydroxyprogesterone caproate, dydrogesterone, and allylestrenol. They are used questionably for treatment of recurrent pregnancy loss and for prevention of preterm birth in pregnant women with a history of at least one spontaneous preterm birth.[42]
Puberty suppression
Progestogens have been used to treat precocious puberty in both boys and girls. They have also been used to delay puberty in transgender youth.
Sexual deviance
Certain progestogens, such as cyproterone acetate and medroxyprogesterone acetate, are used as a form of chemical castration to treat sexual deviance in men, particularly sex offenders. They are specifically used to treat paraphilias and hypersexuality. They work by suppressing gonadal testosterone production and hence circulating testosterone levels. This results in decreased libido and interference with erectile function and ability to attain orgasm.
Skin and hair conditions
Progestogens are used to treat androgen-dependent skin and hair conditions in women. These include oily skin, acne, seborrhea, hirsutism, scalp hair loss, and hidradenitis suppurativa. They act by suppressing testosterone levels and, in the case of antiandrogenic progestogens, by directly blocking the actions of androgens.
Androgen excess
Progestogens are used to treat hyperandrogenism, such as due to polycystic ovary syndrome and congenital adrenal hyperplasia, in women. Examples include cyproterone acetate and chlormadinone acetate.
Appetite stimulation
Certain progestins can be used at very high doses to increase appetite in conditions like cachexia, anorexia, and wasting syndromes. In general, they are used in combination with certain other steroid medications such as dexamethasone. Their effects take several weeks to become apparent, but are relatively long-lived when compared to those of corticosteroids. Furthermore, they are recognized as being the only medications to increase lean body mass. Megestrol acetate is the lead drug of this class for the management of cachexia, and medroxyprogesterone acetate is also used.[43][44] The mechanism of action of the appetite-related effects of these two medications is unknown and may not be related to their progestogenic activity. Very high doses of other progestogens, like cyproterone acetate, have minimal or no influence on appetite and weight.
Contraindications
Contraindications of progestogens may include breast cancer and a history of venous thromboembolism among others.[45][citation needed]
Side effects
Progestogens have relatively few side effects at typical dosages.[46] Side effects of progestogens may include tiredness, dysphoria, depression, mood changes, menstrual irregularities, hypomenorrhea, edema, vaginal dryness, vaginal atrophy, headaches, nausea, breast tenderness, decreased libido.[1][2][46] Progestins with androgenic activity, namely 19-nortestosterone derivatives, can also cause acne, hirsutism, seborrhea, voice deepening, changes in liver protein production (e.g., decreased HDL cholesterol, sex hormone-binding globulin), increased appetite, and weight gain, among others.[1][46] Other side effects of progestogens may include an increased risk of breast cancer, cardiovascular disease, and blood clots, among others.[2] Some of the side effects of progestogens are due not to their progestogenic activity but rather due to off-target activities (e.g., androgenic activity, glucocorticoid activity, antimineralocorticoid activity).[1][47] At high doses, due to their antigonadotropic effects, progestogens can cause low sex hormone levels and associated side effects like diminished secondary sexual characteristics, sexual dysfunction (e.g., reduced sex drive and erectile dysfunction), reversible infertility, reduced bone mineral density, and an increased risk of bone fractures, both in men and in premenopausal women.[3]
| Clinical outcome | Hypothesized effect on risk |
Estrogen and progestogen (CEs 0.625 mg/day p.o. + MPA 2.5 mg/day p.o.) (n = 16,608, with uterus, 5.2–5.6 years follow up) |
Estrogen alone (CEs 0.625 mg/day p.o.) (n = 10,739, no uterus, 6.8–7.1 years follow up) | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | AR | HR | 95% CI | AR | ||
| Coronary heart disease | Decreased | 1.24 | 1.00–1.54 | +6 / 10,000 PYs | 0.95 | 0.79–1.15 | −3 / 10,000 PYs |
| Stroke | Decreased | 1.31 | 1.02–1.68 | +8 / 10,000 PYs | 1.37 | 1.09–1.73 | +12 / 10,000 PYs |
| Pulmonary embolism | Increased | 2.13 | 1.45–3.11 | +10 / 10,000 PYs | 1.37 | 0.90–2.07 | +4 / 10,000 PYs |
| Venous thromboembolism | Increased | 2.06 | 1.57–2.70 | +18 / 10,000 PYs | 1.32 | 0.99–1.75 | +8 / 10,000 PYs |
| Breast cancer | Increased | 1.24 | 1.02–1.50 | +8 / 10,000 PYs | 0.80 | 0.62–1.04 | −6 / 10,000 PYs |
| Colorectal cancer | Decreased | 0.56 | 0.38–0.81 | −7 / 10,000 PYs | 1.08 | 0.75–1.55 | +1 / 10,000 PYs |
| Endometrial cancer | – | 0.81 | 0.48–1.36 | −1 / 10,000 PYs | – | – | – |
| Hip fractures | Decreased | 0.67 | 0.47–0.96 | −5 / 10,000 PYs | 0.65 | 0.45–0.94 | −7 / 10,000 PYs |
| Total fractures | Decreased | 0.76 | 0.69–0.83 | −47 / 10,000 PYs | 0.71 | 0.64–0.80 | −53 / 10,000 PYs |
| Total mortality | Decreased | 0.98 | 0.82–1.18 | −1 / 10,000 PYs | 1.04 | 0.91–1.12 | +3 / 10,000 PYs |
| Global index | – | 1.15 | 1.03–1.28 | +19 / 10,000 PYs | 1.01 | 1.09–1.12 | +2 / 10,000 PYs |
| Diabetes | – | 0.79 | 0.67–0.93 | 0.88 | 0.77–1.01 | ||
| Gallbladder disease | Increased | 1.59 | 1.28–1.97 | 1.67 | 1.35–2.06 | ||
| Stress incontinence | – | 1.87 | 1.61–2.18 | 2.15 | 1.77–2.82 | ||
| Urge incontinence | – | 1.15 | 0.99–1.34 | 1.32 | 1.10–1.58 | ||
| Peripheral artery disease | – | 0.89 | 0.63–1.25 | 1.32 | 0.99–1.77 | ||
| Probable dementia | Decreased | 2.05 | 1.21–3.48 | 1.49 | 0.83–2.66 | ||
| Abbreviations: CEs = conjugated estrogens. MPA = medroxyprogesterone acetate. p.o. = per oral. HR = hazard ratio. AR = attributable risk. PYs = person–years. CI = confidence interval. Notes: Sample sizes (n) include placebo recipients, which were about half of patients. "Global index" is defined for each woman as the time to earliest diagnosis for coronary heart disease, stroke, pulmonary embolism, breast cancer, colorectal cancer, endometrial cancer (estrogen plus progestogen group only), hip fractures, and death from other causes. Sources: See template. | |||||||
Mood changes
Birth control
The available evidence on the risk of mood changes and depression with progestogens in hormonal birth control is limited.[48][49] As of 2019, there is no consistent evidence for adverse effects on mood of hormonal birth control, including progestogen-only birth control and combined birth control, in the general population.[50][51] Most women taking combined birth control experience no influence or a beneficial effect on mood.[48][51][49] Adverse effects on mood appear to be infrequent, occurring only in a small percentage of women.[48][51][49] About 5 to 10% of women experience negative mood changes with combined birth control pills, and about 5% of women discontinue birth control pills due to such changes.[52][48] A study of about 4,000 women found that progestogen-only birth control with depot medroxyprogesterone acetate had an incidence of depression of 1.5% and discontinuation due to depression of 0.5%.[51][53][54] Beneficial effects of hormonal birth control such as decreased menstrual pain and bleeding may positively influence mood.[48]
A 2018 systematic review of 26 studies, including 5 randomized controlled trials and 21 observational studies, found that the overall evidence showed no association between progestogen-only birth control and depression.[51] The progestins assessed included depot medroxyprogesterone acetate, levonorgestrel-containing contraceptive implants and intrauterine devices, and progestogen-only birth control pills.[51] Findings of large observational studies are mixed due to prominent confounding factors, but overall show no association of hormonal birth control with depression.[50][51] Randomized controlled trials typically do not find clinically significant influences of hormonal birth control on mood.[50][51] Reviews from before 1980 reported a high incidence of adverse mood effects with combined birth control pills.[48] However, doses of estrogens and progestogens in birth control pills before 1980 were considerably higher than those used today, and these doses frequently caused unpleasant side effects that may have unfavorably influenced mood.[48][55]
Mood with birth control pills may be better with monophasic and continuous formulations than with triphasic and cyclic formulations.[48][52] Limited and inconsistent evidence supports differences in mood with hormonal birth control using different doses of ethinylestradiol or different routes of administration, such as birth control pills versus contraceptive vaginal rings and contraceptive patches.[48][52] Combined birth control with less androgenic or antiandrogenic progestins like desogestrel, gestodene, and drospirenone may have a more favorable influence on mood than birth control with more androgenic progestins like levonorgestrel.[48][52] However, androgen supplementation with hormonal birth control has also been reported to improve mood.[48]
Hormonal birth control that suppresses ovulation is effective in the treatment of premenstrual dysphoric disorder (PMDD).[50][56] Combined birth control pills containing drospirenone are approved for the treatment of PMDD and may be particularly beneficial due to the antimineralocorticoid activity of drospirenone.[50][57][58] Studies on the influence of hormonal birth control on mood in women with existing mood disorders or polycystic ovary syndrome are limited and mixed.[50][48] Women with underlying mood disorders may be more likely to experience mood changes with hormonal birth control.[48][50][59] A 2016 systematic review found based on limited evidence from 6 studies that hormonal birth control, including combined birth control pills, depot medroxyprogesterone acetate, and levonorgestrel-containing intrauterine devices, was not associated with worse outcomes compared to non-use in women with depressive or bipolar disorders.[60] A 2008 Cochrane review found a greater likelihood of postpartum depression in women given norethisterone enanthate as a form of progestogen-only injectable birth control, and recommended caution on the use of progestogen-only birth control in the postpartum period.[61]
Studies suggest a negativity bias in emotion recognition and reactivity with hormonal birth control.[59] Some data suggests blunted reward responses and potential dysregulation of the stress response with hormonal birth control in some women.[59][50]
Hormone therapy
Estrogen therapy appears to have a beneficial influence on mood in depressed and euthymic perimenopausal women.[62][63][64] Conversely, research on combined estrogen and progestogen therapy for depressive symptoms in menopausal women is scarce and inconclusive.[62][63] Some researchers contend that progestogens have an adverse influence on mood and reduce the benefits of estrogens on mood,[65][66][2] whereas other researchers maintain that progestogens have no adverse influence on mood.[67][68] Progesterone differs from progestins in terms of effects in the brain and might have different effects on mood in comparison.[2][69][1] The available evidence, although limited, suggests no adverse influence of progesterone on mood when used in menopausal hormone therapy.[70]
Sexual function
In most women, sexual desire is unchanged or increased with combined birth control pills.[71] This is despite an increase in sex hormone-binding globulin (SHBG) levels and a decrease in total and free testosterone levels.[71][72] However, findings are conflicting, and more research is needed.[73]
Blood clots
Venous thromboembolism (VTE) consists of deep vein thrombosis (DVT) and pulmonary embolism (PE).[74] DVT is a blood clot in a deep vein, most commonly in the legs, while PE occurs when a clot breaks free and blocks an artery in the lungs.[74] VTE is a rare but potentially fatal cardiovascular event.[74] Estrogens and progestogens can increase coagulation by modulating synthesis of coagulation factors.[1][75][76][77] As a result, they increase the risk of VTE, especially during pregnancy when estrogen and progesterone levels are very high as well as during the postpartum period.[75][76][78] Physiological levels of estrogen and/or progesterone may also influence risk of VTE—with late menopause (≥55 years) being associated with greater risk than early menopause (≤45 years).[79][80]
Progestogen monotherapy
Progestogens when used by themselves at typical clinical dosages, for instance in progestogen-only birth control, do not affect coagulation[81][82][83][84][75][77] and are not generally associated with a higher risk of venous thromboembolism (VTE).[85][86][87][88] An exception is medroxyprogesterone acetate as a progestogen-only injectable contraceptive, which has been associated with a 2- to 4-fold increase in risk of VTE relative to other progestogens and non-use.[89][90][91][92][93][94][88] The reasons for this are unknown, but the observations might be a statistical artifact of preferential prescription of depot medroxyprogesterone acetate to women at risk for VTE.[90] Alternatively, medroxyprogesterone acetate may be an exception among progestogens in terms of influence on VTE risk,[88][92][81][94] possibly due to its partial glucocorticoid activity.[1][6][81] In contrast to depot medroxyprogesterone acetate, no increase in VTE risk has been observed with moderately high doses of the related progestin chlormadinone acetate (10 mg/day for 18–20 days/cycle), though based on limited data.[94][95]
Very-high-dose progestogen therapy, including with medroxyprogesterone acetate, megestrol acetate, and cyproterone acetate, has been associated with activation of coagulation and a dose-dependent increased risk of VTE.[82][87][96][97][98][99] In studies with high-dose cyproterone acetate specifically, the increase in VTE risk has ranged from 3- to 5-fold.[96][98][99] The incidence of VTE in studies with very-high-dose progestogen therapy has been found to range from 2 to 8%.[82][100][101] However, the relevant patient populations, namely aged individuals with cancer, are already predisposed to VTE, and this greatly amplifies the risk.[82][87][102]
Estrogen plus progestogen therapy
In contrast to progestogen-only birth control, the addition of progestins to oral estrogen therapy, including in combined birth control pills and menopausal hormone therapy, is associated with a higher risk of VTE than with oral estrogen therapy alone.[103][104][105][106][107] The risk of VTE is increased by about 2-fold or less with such regimens in menopausal hormone therapy and by 2- to 4-fold with combined birth control pills containing ethinylestradiol, both relative to non-use.[103][76][106][107] In contrast to oral estrogen therapy, parenteral estradiol, such as with transdermal estradiol, is not associated with a higher risk of VTE.[103][92][106] This is likely due to its lack of first-pass effect in the liver.[1][89] Research is mixed on whether addition of progestins to transdermal estradiol is associated with a greater risk of VTE, with some studies finding no increase in risk and others finding higher risk.[103][92][106] Unlike the case of transdermal estradiol, VTE risk is not lower with ethinylestradiol-containing contraceptive vaginal rings and contraceptive patches compared to combined birth control pills with ethinylestradiol.[76][108][81] This is thought to be due to the resistance of ethinylestradiol to hepatic metabolism.[1][109][89][81]
The type of progestin in combined birth control may modulate the risk of VTE.[104][105][94] Studies have found that combined birth control pills containing newer-generation progestins such as desogestrel, gestodene, norgestimate, drospirenone, and cyproterone acetate are associated with a 1.5- to 3-fold higher risk of VTE than birth control pills containing first-generation progestins such as levonorgestrel and norethisterone.[104][105][107][94][110][111] However, although this has been apparent in retrospective cohort and nested case–control studies, no greater risk of VTE has been observed in prospective cohort and case–control studies.[104][105][112][113][107] These kinds of observational studies have certain advantages over the aforementioned types of studies, such as better ability to control for confounding factors like new-user bias.[113][81] As such, it is unclear whether the higher risk of VTE with newer-generation birth control pills is a real finding or a statistical artifact.[113] Androgenic progestins have been found to antagonize to some degree the effect of estrogens on coagulation.[83][84][75][114][81] First-generation progestins are more androgenic, while newer-generation progestins are weakly androgenic or antiandrogenic, and this might explain the observed differences in risk of VTE.[104][115][75][114] The type of estrogen also influences VTE risk.[109][116][117] Birth control pills containing estradiol valerate are associated with about half the VTE risk of birth control pills with ethinylestradiol.[116][117]
The type of progestogen in combined menopausal hormone therapy may also modulate VTE risk.[118][119] Oral estrogens plus dydrogesterone appears to have lower VTE risk relative to inclusion of other progestins.[120][121][106] Norpregnane derivatives such as nomegestrol acetate and promegestone have been associated with a significantly greater risk of VTE than pregnane derivatives such as medroxyprogesterone acetate and dydrogesterone and nortestosterone derivatives such as norethisterone and levonorgestrel.[118][119] However, these findings may just be statistical artifacts.[119] In contrast to progestins, the addition of oral progesterone to either oral or transdermal estrogen therapy is not associated with a higher risk of VTE.[92][122] However, oral progesterone achieves very low progesterone levels and has relatively weak progestogenic effects, which might be responsible for the absence of increase in VTE risk.[122] Parenteral progesterone, such as vaginal or injectable progesterone, which can achieve luteal-phase levels of progesterone and associated progestogenic effects, has not been characterized in terms of VTE risk.[122]
A 2012 meta-analysis estimated that the absolute risk of VTE is 2 per 10,000 women for non-use, 8 per 10,000 women for ethinylestradiol and levonorgestrel-containing birth control pills, and 10 to 15 per 10,000 women for birth control pills containing ethinylestradiol and a newer-generation progestin.[76] For comparison, the absolute risk of VTE is generally estimated as 1 to 5 per 10,000 woman-years for non-use, 5 to 20 per 10,000 woman-years for pregnancy, and 40 to 65 per 10,000 woman-years for the postpartum period.[76] Risk of VTE with estrogen and progestogen therapy is highest at the start of treatment, particularly during the first year, and decreases over time.[89][123] Older age, higher body weight, lower physical activity, and smoking are all associated with a higher risk of VTE with oral estrogen and progestogen therapy.[89][122][123][124] Women with thrombophilia have a dramatically higher risk of VTE with estrogen and progestogen therapy than women without thrombophilia.[76][108] Depending on the condition, risk of VTE can be increased as much as 50-fold in such women relative to non-use.[76][108]
Estrogens induce the production of sex hormone-binding globulin (SHBG) in the liver.[1][81] As such, SHBG levels indicate hepatic estrogenic exposure and may be a reliable surrogate marker for coagulation and VTE risk with estrogen therapy.[125][126][127] Combined birth control pills containing different progestins result in SHBG levels that are increased 1.5- to 2-fold with levonorgestrel, 2.5- to 4-fold with desogestrel and gestodene, 3.5- to 4-fold with drospirenone and dienogest, and 4- to 5-fold with cyproterone acetate.[125] SHBG levels differ depending on the progestin because androgenic progestins oppose the effect of ethinylestradiol on hepatic SHBG production as with its procoagulatory effects.[1][81] Contraceptive vaginal rings and contraceptive patches likewise have been found to increase SHBG levels by 2.5-fold and 3.5-fold, respectively.[125][81] Birth control pills containing high doses of ethinylestradiol (>50 μg) can increase SHBG levels by 5- to 10-fold, which is similar to the increase that occurs during pregnancy.[128] Conversely, increases in SHBG levels are much lower with estradiol, especially when it is used parenterally.[129][130][131][132][133] Estradiol-containing combined birth control pills, like estradiol valerate/dienogest and estradiol/nomegestrol acetate, and high-dose parenteral polyestradiol phosphate therapy have both been found to increase SHBG levels by about 1.5-fold.[81][134][132][131]
Hormone therapy with high-dose ethinylestradiol and cyproterone acetate in transgender women has been associated with a 20- to 45-fold higher risk of VTE relative to non-use.[102][123] The absolute incidence was about 6%.[102][123] Conversely, the risk of VTE in transgender women is much lower with oral or transdermal estradiol plus high-dose cyproterone acetate.[102][123] Ethinylestradiol is thought to have been primarily responsible for the VTE risk, but cyproterone acetate may have contributed as well.[102] Ethinylestradiol is no longer used in transgender hormone therapy,[135][136][137] and doses of cyproterone acetate have been reduced.[138][139]
| Type | Route | Medications | Odds ratio (95% CI) |
|---|---|---|---|
| Menopausal hormone therapy | Oral | Estradiol alone ≤1 mg/day >1 mg/day |
1.27 (1.16–1.39)* 1.22 (1.09–1.37)* 1.35 (1.18–1.55)* |
| Conjugated estrogens alone ≤0.625 mg/day >0.625 mg/day |
1.49 (1.39–1.60)* 1.40 (1.28–1.53)* 1.71 (1.51–1.93)* | ||
| Estradiol/medroxyprogesterone acetate | 1.44 (1.09–1.89)* | ||
| Estradiol/dydrogesterone ≤1 mg/day E2 >1 mg/day E2 |
1.18 (0.98–1.42) 1.12 (0.90–1.40) 1.34 (0.94–1.90) | ||
| Estradiol/norethisterone ≤1 mg/day E2 >1 mg/day E2 |
1.68 (1.57–1.80)* 1.38 (1.23–1.56)* 1.84 (1.69–2.00)* | ||
| Estradiol/norgestrel or estradiol/drospirenone | 1.42 (1.00–2.03) | ||
| Conjugated estrogens/medroxyprogesterone acetate | 2.10 (1.92–2.31)* | ||
| Conjugated estrogens/norgestrel ≤0.625 mg/day CEEs >0.625 mg/day CEEs |
1.73 (1.57–1.91)* 1.53 (1.36–1.72)* 2.38 (1.99–2.85)* | ||
| Tibolone alone | 1.02 (0.90–1.15) | ||
| Raloxifene alone | 1.49 (1.24–1.79)* | ||
| Transdermal | Estradiol alone ≤50 μg/day >50 μg/day |
0.96 (0.88–1.04) 0.94 (0.85–1.03) 1.05 (0.88–1.24) | |
| Estradiol/progestogen | 0.88 (0.73–1.01) | ||
| Vaginal | Estradiol alone | 0.84 (0.73–0.97) | |
| Conjugated estrogens alone | 1.04 (0.76–1.43) | ||
| Combined birth control | Oral | Ethinylestradiol/norethisterone | 2.56 (2.15–3.06)* |
| Ethinylestradiol/levonorgestrel | 2.38 (2.18–2.59)* | ||
| Ethinylestradiol/norgestimate | 2.53 (2.17–2.96)* | ||
| Ethinylestradiol/desogestrel | 4.28 (3.66–5.01)* | ||
| Ethinylestradiol/gestodene | 3.64 (3.00–4.43)* | ||
| Ethinylestradiol/drospirenone | 4.12 (3.43–4.96)* | ||
| Ethinylestradiol/cyproterone acetate | 4.27 (3.57–5.11)* | ||
| Notes: (1) Nested case–control studies (2015, 2019) based on data from the QResearch and Clinical Practice Research Datalink (CPRD) databases. (2) Bioidentical progesterone was not included, but is known to be associated with no additional risk relative to estrogen alone. Footnotes: * = Statistically significant (p < 0.01). Sources: See template. | |||
Cardiovascular health
Progestogens may influence the risk of cardiovascular disease in women.[118] In the women's Health Initiative (WHI), the risk of coronary heart disease was greater with the combination of estrogen plus a progestin (specifically medroxyprogesterone acetate) than with estrogen alone.[140][141][142] However, progestogens have varying activities and may differ in terms of cardiovascular risk.[118][143][144][145][146][147] A 2015 Cochrane review provided strong evidence that the treatment of post-menopausal women with hormone therapy for cardiovascular disease had little if any effect and increased the risk of stroke and venous thromboembolic events.[148] It is thought that androgenic progestins like medroxyprogesterone acetate and norethisterone may antagonize the beneficial effects of estrogens on biomarkers of cardiovascular health (e.g., favorable lipid profile changes).[118][149] However, these findings are mixed and controversial.[149] Differences of progestogens on cardiovascular health and risk have been reviewed and summarized:[118]
- "Unfortunately, there are few long-term clinical studies comparing different progestogens used in [hormone therapy] with respect to cardiovascular outcomes. However, some aspects of potential cardiovascular risk have been examined, namely effects on lipids, vascular function/blood pressure, inflammation, thrombosis, and carbohydrate metabolism. [...] Although progestins have differing effects on aspects of cardiovascular risk, in general, those more similar to progesterone have been associated with a lower impact than the more androgenic progestins on the beneficial effects of concomitant estrogen therapy. However, the limited number of long-term clinical studies makes it difficult to extrapolate the short-term effects on various markers of cardiovascular risk to long-term cardiovascular morbidity."[118]
Route of administration might also influence the cardiovascular health effects of progestogens, but more research is needed similarly.[150]
Breast cancer
Estrogen alone, progestogen alone, and combined estrogen and progestogen therapy are all associated with increased risks of breast cancer when used in menopausal hormone therapy for peri- and postmenopausal women relative to non-use.[151][152][153] These risks are higher for combined estrogen and progestogen therapy than with estrogen alone or progestogen alone.[151][153] In addition to breast cancer risk, estrogen alone and estrogen plus progestogen therapy are associated with higher breast cancer mortality.[154] With 20 years of use, breast cancer incidence is about 1.5-fold higher with estrogen alone and about 2.5-fold higher with estrogen plus progestogen therapy relative to non-use.[151] The increase in breast cancer risk with estrogen and progestogen therapy was shown to be causal with conjugated estrogens plus medroxyprogesterone acetate in the Women's Health Initiative randomized controlled trials.[122][155]
Breast cancer risk with combined estrogen and progestogen therapy may differ depending on the progestogen used.[152][151][118][156] Progestins including chlormadinone acetate, cyproterone acetate, medrogestone, medroxyprogesterone acetate, nomegestrol acetate, norethisterone acetate, promegestone, and tibolone have all been associated with similarly increased risk of breast cancer.[156][152][151] Some research has found that oral progesterone and dydrogesterone with short-term use (<5 years) may be associated with lower risk of breast cancer relative to other progestins.[152][151][118][156] In the long-term however (>5 years), oral progesterone and dydrogesterone have been associated with significantly increased breast cancer risk similarly to other progestogens.[151][157] The lower risk of breast cancer with oral progesterone than with other progestogens may be related to the very low progesterone levels and relatively weak progestogenic effects it produces.[158][122][6]
The risk of breast cancer with estrogen and progestogen therapy in peri- and postmenopausal women is dependent on the duration of treatment, with more than 5 years of use being associated with significantly greater risk than less than five years of use.[151][152] In addition, continuous estrogen and progestogen therapy is associated with a higher risk of breast cancer than cyclic use.[151][152]
A nationwide observational study found that transfeminine hormone therapy with estrogen plus high-dose cyproterone acetate was associated with a 46-fold increased risk of breast cancer in transgender women relative to the expected incidence for cisgender men.[159][160][161][162] However, the risk of breast cancer was still lower than that in cisgender women.[159][160][161][162] The extent to which the increase in breast cancer risk was related to estrogen versus cyproterone acetate is unknown.[159][160][161][162]
| Therapy | <5 years | 5–14 years | 15+ years | |||
|---|---|---|---|---|---|---|
| Cases | RR (95% CI) | Cases | RR (95% CI) | Cases | RR (95% CI) | |
| Estrogen alone | 1259 | 1.18 (1.10–1.26) | 4869 | 1.33 (1.28–1.37) | 2183 | 1.58 (1.51–1.67) |
| By estrogen | ||||||
| Conjugated estrogens | 481 | 1.22 (1.09–1.35) | 1910 | 1.32 (1.25–1.39) | 1179 | 1.68 (1.57–1.80) |
| Estradiol | 346 | 1.20 (1.05–1.36) | 1580 | 1.38 (1.30–1.46) | 435 | 1.78 (1.58–1.99) |
| Estropipate (estrone sulfate) | 9 | 1.45 (0.67–3.15) | 50 | 1.09 (0.79–1.51) | 28 | 1.53 (1.01–2.33) |
| Estriol | 15 | 1.21 (0.68–2.14) | 44 | 1.24 (0.89–1.73) | 9 | 1.41 (0.67–2.93) |
| Other estrogens | 15 | 0.98 (0.46–2.09) | 21 | 0.98 (0.58–1.66) | 5 | 0.77 (0.27–2.21) |
| By route | ||||||
| Oral estrogens | – | – | 3633 | 1.33 (1.27–1.38) | – | – |
| Transdermal estrogens | – | – | 919 | 1.35 (1.25–1.46) | – | – |
| Vaginal estrogens | – | – | 437 | 1.09 (0.97–1.23) | – | – |
| Estrogen and progestogen | 2419 | 1.58 (1.51–1.67) | 8319 | 2.08 (2.02–2.15) | 1424 | 2.51 (2.34–2.68) |
| By progestogen | ||||||
| (Levo)norgestrel | 343 | 1.70 (1.49–1.94) | 1735 | 2.12 (1.99–2.25) | 219 | 2.69 (2.27–3.18) |
| Norethisterone acetate | 650 | 1.61 (1.46–1.77) | 2642 | 2.20 (2.09–2.32) | 420 | 2.97 (2.60–3.39) |
| Medroxyprogesterone acetate | 714 | 1.64 (1.50–1.79) | 2012 | 2.07 (1.96–2.19) | 411 | 2.71 (2.39–3.07) |
| Dydrogesterone | 65 | 1.21 (0.90–1.61) | 162 | 1.41 (1.17–1.71) | 26 | 2.23 (1.32–3.76) |
| Progesterone | 11 | 0.91 (0.47–1.78) | 38 | 2.05 (1.38–3.06) | 1 | – |
| Promegestone | 12 | 1.68 (0.85–3.31) | 19 | 2.06 (1.19–3.56) | 0 | – |
| Nomegestrol acetate | 8 | 1.60 (0.70–3.64) | 14 | 1.38 (0.75–2.53) | 0 | – |
| Other progestogens | 12 | 1.70 (0.86–3.38) | 19 | 1.79 (1.05–3.05) | 0 | – |
| By progestogen frequency | ||||||
| Continuous | – | – | 3948 | 2.30 (2.21–2.40) | – | – |
| Intermittent | – | – | 3467 | 1.93 (1.84–2.01) | – | – |
| Progestogen alone | 98 | 1.37 (1.08–1.74) | 107 | 1.39 (1.11–1.75) | 30 | 2.10 (1.35–3.27) |
| By progestogen | ||||||
| Medroxyprogesterone acetate | 28 | 1.68 (1.06–2.66) | 18 | 1.16 (0.68–1.98) | 7 | 3.42 (1.26–9.30) |
| Norethisterone acetate | 13 | 1.58 (0.77–3.24) | 24 | 1.55 (0.88–2.74) | 6 | 3.33 (0.81–13.8) |
| Dydrogesterone | 3 | 2.30 (0.49–10.9) | 11 | 3.31 (1.39–7.84) | 0 | – |
| Other progestogens | 8 | 2.83 (1.04–7.68) | 5 | 1.47 (0.47–4.56) | 1 | – |
| Miscellaneous | ||||||
| Tibolone | – | – | 680 | 1.57 (1.43–1.72) | – | – |
| Notes: Meta-analysis of worldwide epidemiological evidence on menopausal hormone therapy and breast cancer risk by the Collaborative Group on Hormonal Factors in Breast Cancer (CGHFBC). Fully adjusted relative risks for current versus never-users of menopausal hormone therapy. Source: See template. | ||||||
| Study | Therapy | Hazard ratio (95% CI) |
|---|---|---|
| E3N-EPIC: Fournier et al. (2005) | Estrogen alone | 1.1 (0.8–1.6) |
| Estrogen plus progesterone Transdermal estrogen Oral estrogen |
0.9 (0.7–1.2) 0.9 (0.7–1.2) No events | |
| Estrogen plus progestin Transdermal estrogen Oral estrogen |
1.4 (1.2–1.7) 1.4 (1.2–1.7) 1.5 (1.1–1.9) | |
| E3N-EPIC: Fournier et al. (2008) | Oral estrogen alone | 1.32 (0.76–2.29) |
| Oral estrogen plus progestogen Progesterone Dydrogesterone Medrogestone Chlormadinone acetate Cyproterone acetate Promegestone Nomegestrol acetate Norethisterone acetate Medroxyprogesterone acetate |
Not analyzeda 0.77 (0.36–1.62) 2.74 (1.42–5.29) 2.02 (1.00–4.06) 2.57 (1.81–3.65) 1.62 (0.94–2.82) 1.10 (0.55–2.21) 2.11 (1.56–2.86) 1.48 (1.02–2.16) | |
| Transdermal estrogen alone | 1.28 (0.98–1.69) | |
| Transdermal estrogen plus progestogen Progesterone Dydrogesterone Medrogestone Chlormadinone acetate Cyproterone acetate Promegestone Nomegestrol acetate Norethisterone acetate Medroxyprogesterone acetate |
1.08 (0.89–1.31) 1.18 (0.95–1.48) 2.03 (1.39–2.97) 1.48 (1.05–2.09) Not analyzeda 1.52 (1.19–1.96) 1.60 (1.28–2.01) Not analyzeda Not analyzeda | |
| E3N-EPIC: Fournier et al. (2014) | Estrogen alone | 1.17 (0.99–1.38) |
| Estrogen plus progesterone or dydrogesterone | 1.22 (1.11–1.35) | |
| Estrogen plus progestin | 1.87 (1.71–2.04) | |
| CECILE: Cordina-Duverger et al. (2013) | Estrogen alone | 1.19 (0.69–2.04) |
| Estrogen plus progestogen Progesterone Progestins Progesterone derivatives Testosterone derivatives |
1.33 (0.92–1.92) 0.80 (0.44–1.43) 1.72 (1.11–2.65) 1.57 (0.99–2.49) 3.35 (1.07–10.4) | |
| Footnotes: a = Not analyzed, fewer than 5 cases. Sources: See template. | ||
| Study | Therapy | Hazard ratio (95% CI) |
|---|---|---|
| E3N-EPIC: Fournier et al. (2005)a | Transdermal estrogen plus progesterone <2 years 2–4 years ≥4 years |
0.9 (0.6–1.4) 0.7 (0.4–1.2) 1.2 (0.7–2.0) |
| Transdermal estrogen plus progestin <2 years 2–4 years ≥4 years |
1.6 (1.3–2.0) 1.4 (1.0–1.8) 1.2 (0.8–1.7) | |
| Oral estrogen plus progestin <2 years 2–4 years ≥4 years |
1.2 (0.9–1.8) 1.6 (1.1–2.3) 1.9 (1.2–3.2) | |
| E3N-EPIC: Fournier et al. (2008) | Estrogen plus progesterone <2 years 2–4 years 4–6 years ≥6 years |
0.71 (0.44–1.14) 0.95 (0.67–1.36) 1.26 (0.87–1.82) 1.22 (0.89–1.67) |
| Estrogen plus dydrogesterone <2 years 2–4 years 4–6 years ≥6 years |
0.84 (0.51–1.38) 1.16 (0.79–1.71) 1.28 (0.83–1.99) 1.32 (0.93–1.86) | |
| Estrogen plus other progestogens <2 years 2–4 years 4–6 years ≥6 years |
1.36 (1.07–1.72) 1.59 (1.30–1.94) 1.79 (1.44–2.23) 1.95 (1.62–2.35) | |
| E3N-EPIC: Fournier et al. (2014) | Estrogens plus progesterone or dydrogesterone <5 years ≥5 years |
1.13 (0.99–1.29) 1.31 (1.15–1.48) |
| Estrogen plus other progestogens <5 years ≥5 years |
1.70 (1.50–1.91) 2.02 (1.81–2.26) | |
| Footnotes: a = Oral estrogen plus progesterone was not analyzed because there was a low number of women who used this therapy. Sources: See template. | ||
Overdose
Progestogens are relatively safe in acute overdose.[citation needed]
Interactions
Inhibitors and inducers of cytochrome P450 enzymes and other enzymes such as 5α-reductase may interact with progestogens.[citation needed]
Pharmacology
Pharmacodynamics
Progestogens act by binding to and activating the progesterone receptors (PRs), including the PR-A, PR-B, and PR-C.[1][163][164] Major tissues affected by progestogens include the uterus, cervix, vagina, breasts, and brain.[1] By activating PRs in the hypothalamus and pituitary gland, progestogens suppress the secretion of gonadotropins and thereby function as antigonadotropins at sufficiently high doses.[1] Progesterone interacts with membrane progesterone receptors, but interaction of progestins with these receptors is less clear.[165][166] In addition to their progestogenic activity, many progestogens have off-target activities such as androgenic, antiandrogenic, estrogenic, glucocorticoid, and antimineralocorticoid activity.[1][2][47]
Progestogens mediate their contraceptive effects in women both by inhibiting ovulation (via their antigonadotropic effects) and by thickening cervical mucus, thereby preventing the possibility of fertilization of the ovum by sperm.[4][5] Progestogens have functional antiestrogenic effects in various tissues like the endometrium via activation of the PR, and this underlies their use in menopausal hormone therapy (to prevent unopposed estrogen-induced endometrial hyperplasia and endometrial cancer).[1] The PRs are induced in the breasts by estrogens, and for this reason, it is assumed that progestogens cannot mediate breast changes in the absence of estrogens.[167] The off-target activities of progestogens can contribute both to their beneficial effects and to their adverse effects.[1][2][58]
| Progestogen | Class | Off-target activities | Relative binding affinities (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ES | AN | AA | GC | AM | PR | AR | ER | GR | MR | SHBG | CBG | |||
| Allylestrenola | Estrane | – | ± | – | – | – | 1 | 0 | 0 | 0 | ? | 0 | ? | |
| Chlormadinone acetate | Pregnane | – | – | + | + | – | 67 | 5 | 0 | 8 | 0 | 0 | 0 | |
| Cyproterone acetate | Pregnane | – | – | ++ | + | – | 90 | 6 | 0 | 6 | 8 | 0 | 0 | |
| Demegestone | Norpregnane | – | – | – | – | – | 115 | 1 | 0 | 5 | 1–2 | ? | ? | |
| Desogestrela | Gonane | – | + | – | ± | – | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Dienogest | Gonane | – | – | + | – | – | 5 | 10 | 0 | 1 | 0 | 0 | 0 | |
| Drospirenone | Spirolactone | – | – | + | – | + | 35 | 65 | 0 | 6 | 230 | 0 | 0 | |
| Dydrogesteronea | Pregnane | – | – | – | – | ± | 75 | 0 | ? | ? | ? | ? | ? | |
| Ethisterone | Androstane | – | + | – | – | – | 18 | 0 | 0 | 0 | 0 | ? | ? | |
| Etonogestrel | Gonane | – | + | – | ± | – | 150 | 20 | 0 | 14 | 0 | 15 | 0 | |
| Etynodiola,b | Estrane | + | + | – | – | – | 1 | 0 | 11–18 | 0 | ? | ? | ? | |
| Etynodiol diacetatea | Estrane | + | + | – | – | – | 1 | 0 | 0 | 0 | 0 | ? | ? | |
| Gestodene | Gonane | – | + | – | + | + | 90–432 | 85 | 0 | 27–38 | 97–290 | 40 | 0 | |
| Gestonorone caproate | Pregnane | – | – | – | – | – | ? | ? | ? | ? | ? | ? | ? | |
| Hydroxyprogesterone caproate | Pregnane | – | – | – | – | ± | ? | ? | ? | ? | ? | ? | ? | |
| Levonorgestrel | Gonane | – | + | – | – | – | 150–162 | 45 | 0 | 1–8 | 17–75 | 50 | 0 | |
| Lynestrenola | Estrane | + | + | – | – | – | 1 | 1 | 3 | 0 | 0 | ? | ? | |
| Medrogestone | Pregnane | – | – | ± | – | – | ? | ? | ? | ? | ? | ? | ? | |
| Medroxyprogesterone acetate | Pregnane | – | ± | – | + | – | 115–149 | 5 | 0 | 29–58 | 3–160 | 0 | 0 | |
| Megestrol acetate | Pregnane | – | ± | + | + | – | 65 | 5 | 0 | 30 | 0 | 0 | 0 | |
| Nomegestrol acetate | Norpregnane | – | – | + | – | – | 125 | 42 | 0 | 6 | 0 | 0 | 0 | |
| Norelgestromin | Gonane | – | ± | – | – | – | 10 | 0 | ? | ? | ? | 0 | ? | |
| Norethisterone | Estrane | + | + | – | – | – | 67–75 | 15 | 0 | 0–1 | 0–3 | 16 | 0 | |
| Norethisterone acetatea | Estrane | + | + | – | – | – | 20 | 5 | 1 | 0 | 0 | ? | ? | |
| Norethisterone enanthatea | Estrane | + | + | – | – | – | ? | ? | ? | ? | ? | ? | ? | |
| Noretynodrela | Estrane | + | ± | – | – | – | 6 | 0 | 2 | 0 | 0 | 0 | 0 | |
| Norgestimatea | Gonane | – | + | – | – | – | 15 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Progesterone | Pregnane | – | – | ± | + | + | 50 | 0 | 0 | 10 | 100 | 0 | 36 | |
| Promegestonea | Norpregnane | – | – | – | + | – | 100 | 0 | 0 | 5 | 53 | 0 | 0 | |
| Segesterone acetate | Norpregnane | – | – | – | – | – | 136 | 0 | 0 | 38 | ? | 0 | ? | |
| Tibolonea | Estrane | + | ++ | – | – | – | 6 | 6 | 1 | ? | ? | ? | ||
| Δ4-Tiboloneb | Estrane | – | ++ | – | – | – | 90 | 35 | 1 | 0 | 2 | 1 | 0 | |
| Trimegestone | Norpregnane | – | – | ± | – | ± | 294–330 | 1 | 0 | 9–13 | 42–120 | ? | ? | |
| Footnotes: a = Prodrug. b = Metabolite (non-marketed). Class: Pregnane = Progesterone derivative. Norpregnane = 19-Norprogesterone derivative. Androstane = Testosterone derivative. Estrane = 19-Nortestosterone derivative. Gonane = 13β-Ethylgonane = 18-Methyl-19-nortestosterone derivative. Spirolactone = Spirolactone derivative. Magnitude: ++ = High. + = Moderate. ± = Low. – = None. Activity: ES = Estrogenic. AN = Androgenic. AA = Antiandrogenic. GC = Glucocorticoid. AM = Antimineralocorticoid. Binding: PR: Promegestone = 100%. AR: Metribolone = 100%. ER: Estradiol = 100%. GR: Dexamethasone = 100%. MR: Aldosterone = 100%. SHBG: DHT = 100%. CBG: Cortisol = 100%. Sources: See template. | ||||||||||||||
| Compound | Doses for specific uses (mg/day)[a] | |||||||
|---|---|---|---|---|---|---|---|---|
| OID | TFD | MDT | BCPD | ECD | ||||
| Cycle | Daily | |||||||
| Allylestrenol | 25 | 150–300 | – | 30 | – | – | ||
| Bromoketoprogesterone[b] | – | – | 100–160 | – | – | – | ||
| Chlormadinone acetate | 1.5–4.0 | 20–30 | 3–10 | 1.0–4.0 | 2.0 | 5–10 | ||
| Cyproterone acetate | 1.0 | 20–30 | 1.0–3.0 | 1.0–4.0 | 2.0 | 1.0 | ||
| Desogestrel | 0.06 | 0.4–2.5 | 0.15 | 0.25 | 0.15 | 0.15 | ||
| Dienogest | 1.0 | 6.0–6.3 | – | – | 2.0–3.0 | 2.0 | ||
| Drospirenone | 2.0 | 40–80 | – | – | 3.0 | 2.0 | ||
| Dydrogesterone | >30 | 140–200 | 10–20 | 20 | – | 10 | ||
| Ethisterone | – | 200–700 | 50–250 | – | – | – | ||
| Etynodiol diacetate | 2.0 | 10–15 | – | 1.0 | 1.0–20 | – | ||
| Gestodene | 0.03 | 2.0–3.0 | – | – | 0.06–0.075 | 0.20 | ||
| Hydroxyprogest. acetate | – | – | 70–125 | – | 100 | – | ||
| Hydroxyprogest. caproate | – | 700–1400 | 70 | – | – | – | ||
| Levonorgestrel | 0.05 | 2.5–6.0 | 0.15–0.25 | 0.5 | 0.1–0.15 | 0.075 | ||
| Lynestrenol | 2.0 | 35–150 | 5.0 | 10 | – | – | ||
| Medrogestone | 10 | 50–100 | 10 | 15 | – | 10 | ||
| Medroxyprogest. acetate | 10 | 40–120 | 2.5–10 | 20–30 | 5–10 | 5.0 | ||
| Megestrol acetate | >5[c] | 30–70 | – | 5–10 | 1.0–5.0 | 5.0 | ||
| Nomegestrol acetate | 1.25–5.0 | 100 | 5.0 | – | 2.5 | 3.75–5.0 | ||
| Norethandrolone[b] | – | – | 10 | – | – | – | ||
| Norethisterone | 0.4–0.5 | 100–150 | 5–10 | 10–15 | 0.5 | 0.7–1.0 | ||
| Norethisterone acetate | 0.5 | 30–60 | 2.5–5.0 | 7.5 | 0.6 | 1.0 | ||
| Norethist. acetate (micron.) | – | 12–14 | – | – | – | – | ||
| Noretynodrel | 4.0 | 150–200 | – | 14 | 2.5–10 | – | ||
| Norgestimate | 0.2 | 2.0–10 | – | – | 0.25 | 0.09 | ||
| Norgestrel | 0.1 | 12 | – | 0.5–2.0 | – | – | ||
| Normethandrone | – | 150 | 10 | – | – | – | ||
| Progesterone (non-micron.) | >300[d] | – | – | – | – | – | ||
| Progesterone (micronized) | – | 4200 | 200–300 | 1000 | – | 200 | ||
| Promegestone | 0.5 | 10 | 0.5 | – | – | 0.5 | ||
| Tibolone | 2.5 | – | – | – | – | – | ||
| Trengestone | – | 50–70 | – | – | – | – | ||
| Trimegestone | 0.5 | – | 0.25–0.5 | – | – | 0.0625–0.5 | ||
Notes and sources
| ||||||||
| Compound | Form | Dose for specific uses (mg)[c] | DOA[d] | |||
|---|---|---|---|---|---|---|
| TFD[e] | POICD[f] | CICD[g] | ||||
| Algestone acetophenide | Oil soln. | – | – | 75–150 | 14–32 d | |
| Gestonorone caproate | Oil soln. | 25–50 | – | – | 8–13 d | |
| Hydroxyprogest. acetate[h] | Aq. susp. | 350 | – | – | 9–16 d | |
| Hydroxyprogest. caproate | Oil soln. | 250–500[i] | – | 250–500 | 5–21 d | |
| Medroxyprog. acetate | Aq. susp. | 50–100 | 150 | 25 | 14–50+ d | |
| Megestrol acetate | Aq. susp. | – | – | 25 | >14 d | |
| Norethisterone enanthate | Oil soln. | 100–200 | 200 | 50 | 11–52 d | |
| Progesterone | Oil soln. | 200[i] | – | – | 2–6 d | |
| Aq. soln. | ? | – | – | 1–2 d | ||
| Aq. susp. | 50–200 | – | – | 7–14 d | ||
|
Notes and sources:
| ||||||
Antigonadotropic effects
Progestogens, similarly to the androgens and estrogens through their own respective receptors, inhibit the secretion of the gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH) via activation of the PR in the pituitary gland. This effect is a form of negative feedback on the hypothalamic–pituitary–gonadal axis (HPG axis) and takes advantage of the mechanism that the body uses to prevent sex hormone levels from becoming too high.[215][216][217] Accordingly, progestogens, both endogenous and exogenous (i.e., progestins), have antigonadotropic effects,[218] and progestogens in sufficiently high amounts can markedly suppress the body's normal production of progestogens, androgens, and estrogens as well as inhibit fertility (ovulation in women and spermatogenesis in men).[217]
Progestogens have been found to maximally suppress circulating testosterone levels in men by up to 70 to 80% at sufficiently high doses.[219][220] This is notably less than that achieved by GnRH analogues, which can effectively abolish gonadal production of testosterone and suppress circulating testosterone levels by as much as 95%.[221] It is also less than that achieved by high-dose estrogen therapy, which can suppress testosterone levels into the castrate range similarly to GnRH analogues.[222]
The retroprogesterone derivatives dydrogesterone and trengestone are atypical progestogens and unlike all other clinically used progestogens do not have antigonadotropic effects nor inhibit ovulation even at very high doses.[1][223] In fact, trengestone may have progonadotropic effects, and is actually able to induce ovulation, with about a 50% success rate on average.[223] These progestins also show other atypical properties relative to other progestogens, such as a lack of a hyperthermic effect.[1][223]
Androgenic activity
Some progestins have androgenic activity and can produce androgenic side effects such as increased sebum production (oilier skin), acne, and hirsutism (excessive facial/body hair growth), as well as changes in liver protein production.[224][225][226] Only certain progestins are androgenic however, these being the testosterone derivatives and, to a lesser extent, the 17α-hydroxyprogesterone derivatives medroxyprogesterone acetate and megestrol acetate.[227][225][228] No other progestins have such activity (though some, conversely, possess antiandrogenic activity).[225][228] Moreover, the androgenic activity of progestins within the testosterone derivatives also varies, and while some may have high or moderate androgenic activity, others have only low or no such activity.[21][229]
The androgenic activity of androgenic progestins is mediated by two mechanisms: 1) direct binding to and activation of the androgen receptor; and 2) displacement of testosterone from sex hormone-binding globulin (SHBG), thereby increasing free (and thus bioactive) testosterone levels.[230] The androgenic activity of many androgenic progestins is offset by combination with ethinylestradiol, which robustly increases SHBG levels, and most oral contraceptives in fact markedly reduce free testosterone levels and can treat or improve acne and hirsutism.[230] An exception is progestin-only contraceptives, which do not also contain an estrogen.[230]
The relative androgenic activity of testosterone-derivative progestins and other progestins that have androgenic activity can be roughly ranked as follows:
- Very high: danazol, ethisterone, gestrinone, normethandrone, norvinisterone[231][232][233][234]
- High: levonorgestrel, norgestrel, norgestrienone, tibolone[21][229][231][7][235][236][1]
- Moderate: norethisterone and its prodrugs (norethisterone acetate, norethisterone enanthate, etynodiol diacetate, lynestrenol, quingestanol acetate)[237][21][229][235][238]
- Low: desogestrel, etonogestrel, gestodene, norgestimate[235][238][239]
- Very low or negligible: allylestrenol, dimethisterone, medroxyprogesterone acetate, megestrol acetate, norelgestromin, noretynodrel, norgesterone[1][240][241][242][243][244][245][246]
- Antiandrogenic: dienogest, oxendolone[247][1]
The clinical androgenic and anabolic activity of the androgenic progestins listed above is still far lower than that of conventional androgens and anabolic steroids like testosterone and nandrolone esters. As such, they are only generally associated with such effects in women and often only at high doses. In men, due to their concomitant progestogenic activity and by extension antigonadotropic effects, these progestins can have potent functional antiandrogenic effects via suppression of testosterone production and levels.
Antiandrogenic activity
Some progestogens have antiandrogenic activity in addition to their progestogenic activity.[248] These progestogens, with varying degrees of potency as antiandrogens, include chlormadinone acetate, cyproterone acetate, dienogest, drospirenone, medrogestone, megestrol acetate, nomegestrol acetate, osaterone acetate (veterinary), and oxendolone.[248][247][249][250] The relative antiandrogenic activity in animals of some of these progestogens has been ranked as follows: cyproterone acetate (100%) > nomegestrol acetate (90%) > dienogest (30–40%) ≥ chlormadinone acetate (30%) = drospirenone (30%).[1][83] Antiandrogenic activity in certain progestogens may help to improve symptoms of acne, seborrhea, hirsutism, and other androgen-dependent conditions in women.[1][248]
Estrogenic activity
A few progestins have weak estrogenic activity.[1] These include the 19-nortestosterone derivatives norethisterone, noretynodrel, and tibolone, as well as the norethisterone prodrugs[251] norethisterone acetate, norethisterone enanthate, lynestrenol, and etynodiol diacetate.[1] The estrogenic activity of norethisterone and its prodrugs are due to metabolism into ethinylestradiol.[1] High doses of norethisterone and noretynodrel have been associated with estrogenic side effects such as breast enlargement in women and gynecomastia in men, but also with alleviation of menopausal symptoms in postmenopausal women.[252] In contrast, non-estrogenic progestins were not found to be associated with such effects.[252]
Glucocorticoid activity
Some progestogens, mainly certain 17α-hydroxyprogesterone derivatives, have weak glucocorticoid activity.[253] This can result, at sufficiently high doses, in side effects such as symptoms of Cushing's syndrome, steroid diabetes, adrenal suppression and insufficiency, and neuropsychiatric symptoms like depression, anxiety, irritability, and cognitive impairment.[253][254][255] Progestogens with the potential for clinically relevant glucocorticoid effects include the 17α-hydroxyprogesterone derivatives chlormadinone acetate, cyproterone acetate, medroxyprogesterone acetate, megestrol acetate, promegestone, and segesterone acetate and the testosterone derivatives desogestrel, etonogestrel, and gestodene.[1][254][256][257] Conversely, hydroxyprogesterone caproate possesses no such activity, while progesterone itself has very weak glucocorticoid activity.[258][1]
| Steroid | Class | TR (↑)a | GR (%)b |
|---|---|---|---|
| Dexamethasone | Corticosteroid | ++ | 100 |
| Ethinylestradiol | Estrogen | – | 0 |
| Etonogestrel | Progestin | + | 14 |
| Gestodene | Progestin | + | 27 |
| Levonorgestrel | Progestin | – | 1 |
| Medroxyprogesterone acetate | Progestin | + | 29 |
| Norethisterone | Progestin | – | 0 |
| Norgestimate | Progestin | – | 1 |
| Progesterone | Progestogen | + | 10 |
| Footnotes: a = Thrombin receptor (TR) upregulation (↑) in vascular smooth muscle cells (VSMCs). b = RBA (%) for the glucocorticoid receptor (GR). Strength: – = No effect. + = Pronounced effect. ++ = Strong effect. Sources: [259] | |||
Antimineralocorticoid activity
Certain progestogens, including progesterone, drospirenone, and gestodene, as well as to a lesser extent dydrogesterone and trimegestone, have varying degrees of antimineralocorticoid activity.[1][58] Other progestins might also have significant antimineralocorticoid activity.[260] Progesterone itself has potent antimineralocorticoid activity.[1] No clinically used progestogens are known to have mineralocorticoid activity.[1]
Progestins with potent antimineralocorticoid activity like drospirenone may have properties more similar to those of natural progesterone, such as counteraction of cyclical estrogen-induced sodium and fluid retention, edema, and associated weight gain; lowered blood pressure; and possibly improved cardiovascular health.[261][262][263][264]
Neurosteroid activity
Progesterone has neurosteroid activity via metabolism into allopregnanolone and pregnanolone, potent positive allosteric modulators of the GABAA receptor.[1] As a result, it has associated effects such as sedation, somnolence, and cognitive impairment.[1] No progestin is known to have similar such neurosteroid activity or effects.[1] However, promegestone has been found to act as a non-competitive antagonist of the nicotinic acetylcholine receptor similarly to progesterone.[265]
Other activities
Certain progestins have been found to stimulate the proliferation of MCF-7 breast cancer cells in vitro, an action that is independent of the classical PRs and is instead mediated via the progesterone receptor membrane component-1 (PGRMC1).[266] Norethisterone, desogestrel, levonorgestrel, and drospirenone strongly stimulate proliferation and medroxyprogesterone acetate, dienogest, and dydrogesterone weakly stimulate proliferation, whereas progesterone, nomegestrol acetate, and chlormadinone acetate act neutrally in the assay and do not stimulate proliferation.[266][267] It is unclear whether these findings may explain the different risks of breast cancer observed with progesterone, dydrogesterone, and other progestins such as medroxyprogesterone acetate and norethisterone in clinical studies.[268]
Pharmacokinetics
Oral progesterone has very low bioavailability and potency.[1][6][158][122][269] Micronization and dissolution in oil-filled capsules, a formulation known as oral micronized progesterone (OMP), increases the bioavailability of progesterone by several-fold.[269][270] However, the bioavailability of oral micronized progesterone nonetheless remains very low at less than 2.4%.[1][6][158][122][271] Progesterone also has a very short elimination half-life in the circulation of no more than 1.5 hours.[272][1][269] Due to the poor oral activity of oral micronized progesterone, it has relatively weak progestogenic effects.[6][158][122] Administration of progesterone in oil solution by intramuscular injection has a duration of about 2 or 3 days, necessitating frequent injections.[1][273][274][275][276][277] Transdermal administration of progesterone in the form of creams or gels achieves only very low levels of progesterone and weak progestogenic effects.[278][279]
Due to the poor oral activity of progesterone and its short duration with intramuscular injection, progestins were developed in its place both for oral use and for parenteral administration.[280] Orally active progestins have high oral bioavailability in comparison to oral micronized progesterone.[1] Their bioavailability is generally in the range of 60 to 100%.[1] Their elimination half-lives are also much longer than that of progesterone, in the range of 8 to 80 hours.[1] Due mainly to their pharmacokinetic improvements, progestins have oral potency that is up to several orders of magnitude greater than that of oral micronized progesterone.[1] For example, the oral potency of medroxyprogesterone acetate is at least 30-fold that of oral micronized progesterone, while the oral potency of gestodene is at least 10,000-fold that of oral micronized progesterone.[1] Parenterally administered progestins, such as hydroxyprogesterone caproate in oil solution, norethisterone enanthate in oil solution, and medroxyprogesterone acetate in microcrystalline aqueous suspension, have durations in the range of weeks to months.[273][274][275][276][277]
| Progestogen | Class | Dosea | Bioavailability | Half-life | |
|---|---|---|---|---|---|
| Allylestrenol | Estrane | NA | ? | Prodrug | |
| Chlormadinone acetate | Pregnane | 2 mg | ~100% | 80 hours | |
| Cyproterone acetate | Pregnane | 2 mg | ~100% | 54–79 hours | |
| Desogestrel | Gonane | 0.15 mg | 63% | Prodrug | |
| Dienogest | Gonane | 4 mg | 96% | 11–12 hours | |
| Drospirenone | Spirolactone | 3 mg | 66% | 31–33 hours | |
| Dydrogesterone | Pregnane | 10 mg | 28% | 14–17 hours | |
| Etynodiol diacetate | Estrane | NA | ? | Prodrug | |
| Gestodene | Gonane | 0.075 mg | 88–99% | 12–14 hours | |
| Hydroxyprogesterone caproate | Pregnane | ND | – | 8 daysb | |
| Levonorgestrel | Gonane | 0.15–0.25 mg | 90% | 10–13 hours | |
| Lynestrenol | Estrane | NA | ? | Prodrug | |
| Medrogestone | Pregnane | 5 mg | ~100% | 35 hours | |
| Medroxyprogesterone acetate | Pregnane | 10 mg | ~100% | 24 hours | |
| Megestrol acetate | Pregnane | 160 mg | ~100% | 22 hours | |
| Nomegestrol acetate | Pregnane | 2.5 mg | 60% | 50 hours | |
| Norethisterone | Estrane | 1 mg | 64% | 8 hours | |
| Norethisterone acetate | Estrane | NA | ? | Prodrug | |
| Noretynodrel | Estrane | NA | ? | Prodrug | |
| Norgestimate | Gonane | NA | ? | Prodrug | |
| Progesterone (micronized) | Pregnane | 100–200 mg | <2.4% | 5 hours | |
| Promegestone | Pregnane | NA | ? | Prodrug | |
| Tibolone | Estrane | NA | ? | Prodrug | |
| Trimegestone | Pregnane | 0.5 mg | ~100% | 15 hours | |
| Notes: All by oral administration, unless otherwise noted. Footnotes: a = For the listed pharmacokinetic values. b = By intramuscular injection. Sources: See template. | |||||
Chemistry
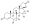
All currently available progestogens are steroidal in terms of chemical structure.[1] Progestogens include the naturally occurring progesterone and the synthetic progestogens (otherwise known as progestins).[1] Progestins can be broadly grouped into two structural classes—chemical derivatives of progesterone and chemical derivatives of testosterone.[1] Progesterone derivatives can be classified into subgroups including pregnanes, retropregnanes, norpregnanes, and spirolactones.[1] Examples of progestins of each of these subgroups include medroxyprogesterone acetate, dydrogesterone, nomegestrol acetate, and drospirenone, respectively.[1] Testosterone derivatives can be classified into subgroups including androstanes, estranes (19-norandrostanes), and gonanes (18-methylestranes).[1][281] Examples of progestins of each of these subgroups include ethisterone, norethisterone, and levonorgestrel, respectively.[1] Many progestins have ester and/or ether substitutions (see progestogen ester) which result in greater lipophilicity and in some cases cause the progestins in question to act as prodrugs in the body.[1]
| Class | Subclass | Progestogen | Structure | Chemical name | Features |
|---|---|---|---|---|---|
| Pregnane | Progesterone | Progesterone | Pregn-4-ene-3,20-dione | – | |
| Quingestrone | Progesterone 3-cyclopentyl enol ether | Ether | |||
| 17α-Hydroxyprogesterone | Acetomepregenol | 3-Deketo-3β,17α-dihydroxy-6-dehydro-6-methylprogesterone 3β,17α-diacetate | Ester | ||
| Algestone acetophenide | 16α,17α-Dihydroxyprogesterone 16α,17α-(cyclic acetal with acetophenone) | Cyclic acetal | |||
| Anagestone acetate | 3-Deketo-6α-methyl-17α-hydroxyprogesterone 17α-acetate | Ester | |||
| Chlormadinone acetate | 6-Dehydro-6-chloro-17α-hydroxyprogesterone 17α-acetate | Ester | |||
| Chlormethenmadinone acetate | 6-Dehydro-6-chloro-16-methylene-17α-hydroxyprogesterone 17α-acetate | Ester | |||
| Cyproterone acetate | 1,2α-Methylene-6-dehydro-6-chloro-17α-hydroxyprogesterone 17α-acetate | Ester; Ring-fused | |||
| Delmadinone acetate | 1,6-Didehydro-6-chloro-17α-hydroxyprogesterone 17α-acetate | Ester | |||
| Flugestone acetate | 9α-Fluoro-11β,17α-dihydroxyprogesterone 17α-acetate | Ester | |||
| Flumedroxone acetate | 6α-(Trifluoromethyl)-17α-hydroxyprogesterone 17α-acetate | Ester | |||
| Hydroxyprogesterone acetate | 17α-Hydroxyprogesterone 17α-acetate | Ester | |||
| Hydroxyprogesterone caproate | 17α-Hydroxyprogesterone 17α-hexanoate | Ester | |||
| Hydroxyprogesterone heptanoate | 17α-Hydroxyprogesterone 17α-heptanoate | Ester | |||
| Medroxyprogesterone acetate | 6α-Methyl-17α-hydroxyprogesterone 17α-acetate | Ester | |||
| Megestrol acetate | 6-Dehydro-6-methyl-17α-hydroxyprogesterone 17α-acetate | Ester | |||
| Melengestrol acetate | 6-Dehydro-6-methyl-16-methylene-17α-hydroxyprogesterone 17α-acetate | Ester | |||
| Methenmadinone acetate | 6-Dehydro-16-methylene-17α-hydroxyprogesterone 17α-acetate | Ester | |||
| Osaterone acetate | 2-Oxa-6-dehydro-6-chloro-17α-hydroxyprogesterone 17α-acetate | Ester | |||
| Pentagestrone acetate | 17α-Hydroxyprogesterone 3-cyclopentyl enol ether 17α-acetate | Ester; Ether | |||
| Proligestone | 14α,17α-Dihydroxyprogesterone 14α,17α-(cyclic acetal with propionaldehyde) | Cyclic acetal | |||
| Other 17α-substituted progesterone | Haloprogesterone | 6α-Fluoro-17α-bromoprogesterone | – | ||
| Medrogestone | 6-Dehydro-6,17α-dimethylprogesterone | – | |||
| Spirolactone | Drospirenone | 6β,7β:15β,16β-Dimethylenespirolactone | Ring-fused | ||
| Norpregnane | 19-Norprogesterone; 17α-Hydroxyprogesterone |
Gestonorone caproate | 17α-Hydroxy-19-norprogesterone 17α-hexanoate | Ester | |
| Nomegestrol acetate | 6-Dehydro-6-methyl-17α-hydroxy-19-norprogesterone 17α-acetate | Ester | |||
| Norgestomet | 11β-Methyl-17α-hydroxy-19-norprogesterone 17α-acetate | Ester | |||
| Segesterone acetate | 16-Methylene-17α-hydroxy-19-norprogesterone 17α-acetate | Ester | |||
| 19-Norprogesterone; Other 17α-substituted progesterone |
Demegestone | 9-Dehydro-17α-methyl-19-norprogesterone | – | ||
| Promegestone | 9-Dehydro-17α,21-dimethyl-19-norprogesterone | – | |||
| Trimegestone | 9-Dehydro-17α,21-dimethyl-19-nor-21β-hydroxyprogesterone | – | |||
| Retropregnane | Retroprogesterone | Dydrogesterone | 6-Dehydro-9β,10α-progesterone | – | |
| Trengestone | 1,6-Didehydro-6-chloro-9β,10α-progesterone | – | |||
| Androstane | 17α-Ethynyltestosterone | Danazol | 2,3-d-Isoxazol-17α-ethynyltestosterone | Ring-fused | |
| Dimethisterone | 6α,21-Dimethyl-17α-ethynyltestosterone | – | |||
| Ethisterone | 17α-Ethynyltestosterone | – | |||
| Estrane | 19-Nortestosterone; 17α-Ethynyltestosterone |
Etynodiol diacetate | 3-Deketo-3β-hydroxy-17α-ethynyl-19-nortestosterone 3β,17β-diacetate | Ester | |
| Lynestrenol | 3-Deketo-17α-ethynyl-19-nortestosterone | – | |||
| Norethisterone | 17α-Ethynyl-19-nortestosterone | – | |||
| Norethisterone acetate | 17α-Ethynyl-19-nortestosterone 17β-acetate | Ester | |||
| Norethisterone enanthate | 17α-Ethynyl-19-nortestosterone 17β-heptanoate | Ester | |||
| Noretynodrel | 5(10)-Dehydro-17α-ethynyl-19-nortestosterone | – | |||
| Norgestrienone | 9,11-Didehydro-17α-ethynyl-19-nortestosterone | – | |||
| Quingestanol acetate | 17α-Ethynyl-19-nortestosterone 3-cyclopentyl enol ether 17β-acetate | Ester; Ether | |||
| Tibolone | 5(10)-Dehydro-7α-methyl-17α-ethynyl-19-nortestosterone | – | |||
| 19-Nortestosterone; Other 17α-substituted testosterone (and 16β-substituted testosterone) |
Allylestrenol | 3-Deketo-17α-allyl-19-nortestosterone | – | ||
| Altrenogest | 9,11-Didehydro-17α-allyl-19-nortestosterone | – | |||
| Dienogest | 9-Dehydro-17α-cyanomethyl-19-nortestosterone | – | |||
| Norgesterone | 5(10)-Dehydro-17α-vinyl-19-nortestosterone | – | |||
| Normethandrone | 17α-Methyl-19-nortestosterone | – | |||
| Norvinisterone | 17α-Vinyl-19-nortestosterone | – | |||
| Oxendolone | 16β-Ethyl-19-nortestosterone | – | |||
| Gonane | 19-Nortestosterone; 17α-Ethynyltestosterone; 18-Methyltestosterone |
Desogestrel | 3-Deketo-11-methylene-17α-ethynyl-18-methyl-19-nortestosterone | – | |
| Etonogestrel | 11-Methylene-17α-ethynyl-18-methyl-19-nortestosterone | – | |||
| Gestodene | 15-Dehydro-17α-ethynyl-18-methyl-19-nortestosterone | – | |||
| Gestrinone | 9,11-Didehydro-17α-ethynyl-18-methyl-19-nortestosterone | – | |||
| Levonorgestrel | 17α-Ethynyl-18-methyl-19-nortestosterone | – | |||
| Norelgestromin | 17α-Ethynyl-18-methyl-19-nortestosterone 3-oxime | Oxime | |||
| Norgestimate | 17α-Ethynyl-18-methyl-19-nortestosterone 3-oxime 17β-acetate | Oxime; Ester | |||
| Norgestrel | rac-13-Ethyl-17α-ethynyl-19-nortestosterone | – |
History
| Generic name | Class[a] | Brand name | Route[b] | Intr. | |
|---|---|---|---|---|---|
| Anagestone acetate | P[i][ii] | Anatropin | PO | 1968 | |
| Chlormethenmadinone acetate | P[i][ii] | Biogest[c] | PO | 1960s | |
| Demegestone | P[iii] | Lutionex | PO | 1974 | |
| Dimethisterone | T[iv] | Lutagan[c] | PO | 1959 | |
| Ethisterone | T[iv] | Pranone[c] | PO, SL | 1939 | |
| Flumedroxone acetate | P[i][ii] | Demigran[c] | PO | 1960s | |
| Haloprogesterone | P[v] | Prohalone | PO | 1961 | |
| Hydroxyprogesterone acetate | P[i][ii] | Prodox | PO | 1957 | |
| Hydroxyprogesterone heptanoate | P[i][ii] | H.O.P.[c] | IM | 1950s | |
| Methenmadinone acetate | P[i][ii] | Superlutin[c] | PO | 1960s | |
| Noretynodrel | T[vi][iv] | Enovid | PO | 1957 | |
| Norgesterone | T[vi][iv] | Vestalin | PO | 1960s | |
| Norgestrienone | T[vi][iv] | Ogyline[c] | PO | 1960s | |
| Norvinisterone | T[vi][iv] | Neoprogestin[c] | PO | 1960s | |
| Pentagestrone acetate | P[i][ii] | Gestovis[c] | PO | 1961 | |
| Quingestanol acetate | T[vi][vii][ii][viii] | Demovis[c] | PO | 1972 | |
| Quingestrone | P[viii] | Enol-Luteovis | PO | 1962 | |
| Trengestone | RP | Retrone | PO | 1974 | |
| |||||
The recognition of progesterone's ability to suppress ovulation during pregnancy spawned a search for a similar hormone that could bypass the problems associated with administering progesterone (e.g. low bioavailability when administered orally and local irritation and pain when continually administered parenterally) and, at the same time, serve the purpose of controlling ovulation. The many synthetic hormones that resulted are known as progestins.
The first orally active progestin, ethisterone (pregneninolone, 17α-ethynyltestosterone), the C17α ethynyl analogue of testosterone, was synthesized in 1938 from dehydroandrosterone by ethynylation, either before or after oxidation of the C3 hydroxyl group, followed by rearrangement of the C5(6) double bond to the C4(5) position. The synthesis was designed by chemists Hans Herloff Inhoffen, Willy Logemann, Walter Hohlweg and Arthur Serini at Schering AG in Berlin and was marketed in Germany in 1939 as Proluton C and by Schering in the U.S. in 1945 as Pranone.[282][283][284][285][286]
A more potent orally active progestin, norethisterone (norethindrone, 19-nor-17α-ethynyltestosterone), the C19 nor analogue of ethisterone, synthesized in 1951 by Carl Djerassi, Luis Miramontes, and George Rosenkranz at Syntex in Mexico City, was marketed by Parke-Davis in the U.S. in 1957 as Norlutin, and was used as the progestin in some of the first oral contraceptives (Ortho-Novum, Norinyl, etc.) in the early 1960s.[283][284][285][286][287]
Noretynodrel, an isomer of norethisterone, was synthesized in 1952 by Frank B. Colton at Searle in Skokie, Illinois and used as the progestin in Enovid, marketed in the U.S. in 1957 and approved as the first oral contraceptive in 1960.[283][284][285][286][288]
Society and culture
Generations
Progestins used in birth control are sometimes grouped, somewhat arbitrarily and inconsistently, into generations. One categorization of these generations is as follows:[14]
- First generation: Approved for marketing before 1973. Examples: noretynodrel, norethisterone (norethindrone), lynestrenol, levonorgestrel.
- Second generation: Approved for marketing between 1973 and 1989. Examples: desogestrel, nomegestrol acetate, norgestimate.
- Third generation: Approved for marketing between 1990 and 2000. Examples: dienogest, etonogestrel.
- Fourth generation: Approved for marketing after 2000. Examples: drospirenone, norelgestromin, segesterone acetate.
Alternatively, estranes such as noretynodrel and norethisterone are classified as first-generation while gonanes such as norgestrel and levonorgestrel are classified as second-generation, with less androgenic gonanes such as desogestrel, norgestimate, and gestodene classified as third-generation and newer progestins like drospirenone classified as fourth-generation.[15] Yet another classification system considers there to be only first- and second-generation progestins.[citation needed]
Classification of progestins by generation has been criticized and it has been argued that the classification scheme should be abandoned.[289]
Availability
Progestogens are available widely throughout the world in many different forms. They are present in all birth control pills.
Etymology
Progestogens, also termed progestagens, progestogens, or gestagens, are compounds which act as agonists of the progesterone receptors.[118][1][143] Progestogens include progesterone—which is the main natural and endogenous progestogen—and progestins, which are synthetic progestogens.[1] Progestins include the 17α-hydroxyprogesterone derivative medroxyprogesterone acetate and the 19-nortestosterone derivative norethisterone, among many other synthetic progestogens.[118][1] As progesterone is a single compound and has no plural form, the term "progesterones" does not exist and is grammatically incorrect.[143] The terms describing progestogens are often confused.[118][143] However, progestogens have differing activities and effects and it is inappropriate to interchange them.[118][1][143]
Research
A variety of progestins have been studied for use as potential male hormonal contraceptives in combination with androgens in men.[290] These include the pregnanes medroxyprogesterone acetate, megestrol acetate, and cyproterone acetate, the norpregnane segesterone acetate, and the estranes norethisterone acetate, norethisterone enanthate, levonorgestrel, levonorgestrel butanoate, desogestrel, and etonogestrel.[290][291][292][293] The androgens that have been used in combination with these progestins include testosterone, testosterone esters, androstanolone (dihydrotestosterone), and nandrolone esters.[290] Dual androgens and progestogens such as trestolone and dimethandrolone undecanoate have also been developed and studied as male contraceptives.[294][295] Doses of progestins used in male hormonal contraception have been noted to be in the range of 5 to 12 times the doses used in female hormonal contraception.[296]
See also
References
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba bb bc bd be bf bg bh bi bj bk bl bm bn bo bp bq br Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- ^ a b c d e f g h Wiegratz I, Kuhl H (August 2004). "Progestogen therapies: differences in clinical effects?". Trends Endocrinol. Metab. 15 (6): 277–85. doi:10.1016/j.tem.2004.06.006. PMID 15358281. S2CID 35891204.
- ^ a b Thibaut F, De La Barra F, Gordon H, Cosyns P, Bradford JM (2010). "The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of paraphilias". World J. Biol. Psychiatry. 11 (4): 604–55. doi:10.3109/15622971003671628. PMID 20459370. S2CID 14949511.
- ^ a b Glasier A (March 20, 2015). "Chapter 134. Contraception". In Jameson JL, De Groot LJ, de Krester D, Giudice LC, Grossman A, Melmed S, Potts Jr JT, Weir GC (eds.). Endocrinology: Adult and Pediatric (7th ed.). Philadelphia: Saunders Elsevier. p. 2306. ISBN 978-0-323-18907-1.
- ^ a b Pattman R, Sankar KN, Elewad B, Handy P, Price DA, eds. (November 19, 2010). "Chapter 33. Contraception including contraception in HIV infection and infection reduction". Oxford Handbook of Genitourinary Medicine, HIV, and Sexual Health (2nd ed.). Oxford: Oxford University Press. p. 360. ISBN 978-0-19-957166-6.
Ovulation may be suppressed in 15–40% of cycles by POPs containing levonorgestrel, norethisterone, or etynodiol diacetate, but in 97–99% by those containing desogestrel.
- ^ a b c d e f g Kuhl H (2011). "Pharmacology of Progestogens" (PDF). J Reproduktionsmed Endokrinol. 8 (1): 157–177.
- ^ a b Christian Lauritzen, John W. W. Studd (22 June 2005). Current Management of the Menopause. CRC Press. p. 45. ISBN 978-0-203-48612-2.
Ethisterone, the first orally effective progestagen, was synthesized by Inhoffen and Hohlweg in 1938. Norethisterone, a progestogen still used worldwide, was synthesized by Djerassi in 1951. But this progestogen was not used immediately and in 1953 Colton discovered norethynodrel, used by Pincus in the first oral contraceptive. Numerous other progestogens were subsequently synthesized, e.g., lynestrenol and ethynodiol diacetate, which were, in fact, prhormones converted in vivo to norethisterone. All these progestogens were also able to induce androgenic effects when high doses were used. More potent progestogens were synthesized in the 1960s, e.g. norgestrel, norgestrienone. These progestogens were also more androgenic.
- ^ Klaus Roth (2014). Chemische Leckerbissen. John Wiley & Sons. p. 69. ISBN 978-3-527-33739-2.
Im Prinzip hatten Hohlweg und Inhoffen die Lösung schon 1938 in der Hand, denn ihr Ethinyltestosteron (11) war eine oral wirksame gestagene Verbindung und Schering hatte daraus bereits 1939 ein Medikament (Proluton C®) entwickelt.
- ^ a b "IBM Watson Health Products: Please Login".
- ^ a b Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. ISBN 978-0-85369-840-1.
- ^ a b "List of Progestins".
- ^ a b Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. ISBN 978-3-88763-075-1.
- ^ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. ISBN 978-1-4757-2085-3.
- ^ a b John David Gordon, Jan Rydfors, Maurice Druzin, Yasser El-Sayed, Yona Tadir (2007). Obstetrics, Gynecology & Infertility: Handbook for Clinicians. Scrub Hill Press, Inc. pp. 229–. ISBN 978-0-9645467-7-6.
- ^ a b Ronald S. Gibbs (2008). Danforth's Obstetrics and Gynecology. Lippincott Williams & Wilkins. pp. 568–. ISBN 978-0-7817-6937-2.
- ^ J. Larry Jameson, Leslie J. De Groot (25 February 2015). Endocrinology: Adult and Pediatric E-Book. Elsevier Health Sciences. pp. 2304–. ISBN 978-0-323-32195-2.
- ^ a b Michelle A. Clark, Richard A. Harvey, Richard Finkel, Jose A. Rey, Karen Whalen (15 December 2011). Pharmacology. Lippincott Williams & Wilkins. p. 322. ISBN 978-1-4511-1314-3.
- ^ a b Bhattacharya (1 January 2003). Pharmacology, 2/e. Elsevier India. p. 378. ISBN 978-81-8147-009-6.
- ^ Rick D. Kellerman, Edward T. Bope (10 November 2017). Conn's Current Therapy 2018 E-Book. Elsevier Health Sciences. pp. 1124–. ISBN 978-0-323-52961-7.
- ^ Helen Varney, Jan M. Kriebs, Carolyn L. Gegor (2004). Varney's Midwifery. Jones & Bartlett Learning. pp. 513–. ISBN 978-0-7637-1856-5.
- ^ a b c d David E. Golan (2008). Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Lippincott Williams & Wilkins. pp. 520–521. ISBN 978-0-7817-8355-2.
- ^ Pamela S. Miles, William F. Rayburn, J.Christopher Carey (6 December 2012). Obstetrics and Gynecology. Springer Science & Business Media. pp. 109–. ISBN 978-1-4684-0220-9.
- ^ a b Erkkola R, Landgren BM (March 2005). "Role of progestins in contraception". Acta Obstet Gynecol Scand. 84 (3): 207–16. doi:10.1111/j.0001-6349.2005.00759.x. PMID 15715527. S2CID 6887415.
- ^ Guise TA, Oefelein MG, Eastham JA, Cookson MS, Higano CS, Smith MR (2007). "Estrogenic side effects of androgen deprivation therapy". Rev Urol. 9 (4): 163–80. PMC 2213888. PMID 18231613.
- ^ Frisk J (2010). "Managing hot flushes in men after prostate cancer--a systematic review". Maturitas. 65 (1): 15–22. doi:10.1016/j.maturitas.2009.10.017. PMID 19962840.
- ^ Koike H, Morikawa Y, Matsui H, Shibata Y, Ito K, Suzuki K (2013). "Chlormadinone acetate is effective for hot flush during androgen deprivation therapy". Prostate Int. 1 (3): 113–6. doi:10.12954/PI.12010. PMC 3814123. PMID 24223412.
- ^ Hickey M, Fraser IS (August 2000). "A functional model for progestogen-induced breakthrough bleeding". Hum. Reprod. 15 (Suppl 3): 1–6. doi:10.1093/humrep/15.suppl_3.1. PMID 11041215.
- ^ a b c Schindler AE (February 2011). "Dydrogesterone and other progestins in benign breast disease: an overview". Archives of Gynecology and Obstetrics. 283 (2): 369–371. doi:10.1007/s00404-010-1456-7. PMID 20383772. S2CID 9125889.
- ^ a b c Winkler UH, Schindler AE, Brinkmann US, Ebert C, Oberhoff C (December 2001). "Cyclic progestin therapy for the management of mastopathy and mastodynia". Gynecological Endocrinology. 15 (Suppl 6): 37–43. doi:10.1080/gye.15.s6.37.43. PMID 12227885. S2CID 27589741.
- ^ a b Ruan X, Mueck AO (November 2014). "Systemic progesterone therapy--oral, vaginal, injections and even transdermal?". Maturitas. 79 (3): 248–255. doi:10.1016/j.maturitas.2014.07.009. PMID 25113944.
- ^ Bińkowska M, Woroń J (June 2015). "Progestogens in menopausal hormone therapy". Przeglad Menopauzalny = Menopause Review. 14 (2): 134–143. doi:10.5114/pm.2015.52154. PMC 4498031. PMID 26327902.
- ^ Kistner RW (1959). "Histological effects of progestins on hyperplasia and carcinoma in situ of the endometrium". Cancer. 12 (6): 1106–22. doi:10.1002/1097-0142(195911/12)12:6<1106::aid-cncr2820120607>3.0.co;2-m. PMID 14409476.
- ^ Regulatory Mechanisms in Transcriptional Signaling. Academic Press. 25 July 2009. pp. 62–. ISBN 978-0-08-091198-4.
- ^ Loren K. Mell, MD (20 December 2011). Gynecologic Cancer. Demos Medical Publishing. pp. 393–. ISBN 978-1-61705-095-4.
- ^ Robert G. McKinnell (13 March 1998). The Biological Basis of Cancer. Cambridge University Press. pp. 262–. ISBN 978-0-521-59695-4.
- ^ Jacqueline Burchum, Laura Rosenthal (2 December 2014). Lehne's Pharmacology for Nursing Care - E-Book. Elsevier Health Sciences. pp. 740–. ISBN 978-0-323-34026-7.
- ^ H. John Smith, Hywel Williams (10 October 2005). Smith and Williams' Introduction to the Principles of Drug Design and Action, Fourth Edition. CRC Press. pp. 493–. ISBN 978-0-203-30415-0.
- ^ a b c d e f David J. Winchester (2006). Breast Cancer. PMPH-USA. pp. 333–. ISBN 978-1-55009-272-1.
- ^ a b Gadducci A, Genazzani AR (December 1999). "Endocrine therapy for gynecological cancer". Gynecol. Endocrinol. 13 (6): 441–56. doi:10.3109/09513599909167590. PMID 10685337.
- ^ a b Lam JS, Leppert JT, Vemulapalli SN, Shvarts O, Belldegrun AS (January 2006). "Secondary hormonal therapy for advanced prostate cancer". J. Urol. 175 (1): 27–34. doi:10.1016/S0022-5347(05)00034-0. PMID 16406864.
- ^ a b Fourcade RO, Chatelain C (July 1998). "Androgen deprivation for prostatic carcinoma: a rationale for choosing components". Int. J. Urol. 5 (4): 303–11. doi:10.1111/j.1442-2042.1998.tb00356.x. PMID 9712436. S2CID 25107178.
- ^ a b Loose, Davis S., Stancel, George M. (2006). "Estrogens and Progestins". In Brunton, Laurence L., Lazo, John S., Parker, Keith L. (eds.). Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill. pp. 1541–71. ISBN 978-0-07-142280-2.
- ^ Maltoni M, Nanni O, Scarpi E, Rossi D, Serra P, Amadori D (March 2001). "High-dose progestins for the treatment of cancer anorexia-cachexia syndrome: a systematic review of randomised clinical trials". Ann. Oncol. 12 (3): 289–300. doi:10.1023/a:1011156811739. PMID 11332139.
- ^ Lelli G, Montanari M, Gilli G, Scapoli D, Antonietti C, Scapoli D (June 2003). "Treatment of the cancer anorexia-cachexia syndrome: a critical reappraisal". J Chemother. 15 (3): 220–5. doi:10.1179/joc.2003.15.3.220. PMID 12868546. S2CID 29442148.
- ^ "IS IT TRUE THAT BIRTH CONTROL PILLS CAUSE BLOOD CLOTS?". National Blood Clot Alliance. Archived from the original on 15 April 2019. Retrieved 15 April 2019.
- ^ a b c Lauritzen C (September 1990). "Clinical use of oestrogens and progestogens". Maturitas. 12 (3): 199–214. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- ^ a b Africander D, Verhoog N, Hapgood JP (June 2011). "Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception". Steroids. 76 (7): 636–52. doi:10.1016/j.steroids.2011.03.001. PMID 21414337. S2CID 23630452.
- ^ a b c d e f g h i j k l m Schaffir J, Worly BL, Gur TL (October 2016). "Combined hormonal contraception and its effects on mood: a critical review". Eur J Contracept Reprod Health Care. 21 (5): 347–55. doi:10.1080/13625187.2016.1217327. PMID 27636867. S2CID 11959163.
- ^ a b c Böttcher B, Radenbach K, Wildt L, Hinney B (July 2012). "Hormonal contraception and depression: a survey of the present state of knowledge". Arch. Gynecol. Obstet. 286 (1): 231–6. doi:10.1007/s00404-012-2298-2. PMID 22467147. S2CID 26204975.
- ^ a b c d e f g h Robakis T, Williams KE, Nutkiewicz L, Rasgon NL (June 2019). "Hormonal Contraceptives and Mood: Review of the Literature and Implications for Future Research". Curr Psychiatry Rep. 21 (7): 57. doi:10.1007/s11920-019-1034-z. PMID 31172309. S2CID 174818119.
- ^ a b c d e f g h Worly BL, Gur TL, Schaffir J (June 2018). "The relationship between progestin hormonal contraception and depression: a systematic review". Contraception. 97 (6): 478–489. doi:10.1016/j.contraception.2018.01.010. PMID 29496297. S2CID 3644828.
- ^ a b c d Poromaa IS, Segebladh B (April 2012). "Adverse mood symptoms with oral contraceptives". Acta Obstet Gynecol Scand. 91 (4): 420–7. doi:10.1111/j.1600-0412.2011.01333.x. PMID 22136510. S2CID 43671664.
- ^ Bakry S, Merhi ZO, Scalise TJ, Mahmoud MS, Fadiel A, Naftolin F (July 2008). "Depot-medroxyprogesterone acetate: an update". Arch. Gynecol. Obstet. 278 (1): 1–12. doi:10.1007/s00404-007-0497-z. PMID 18470526. S2CID 11340062.
- ^ Westhoff C, Truman C, Kalmuss D, Cushman L, Davidson A, Rulin M, Heartwell S (April 1998). "Depressive symptoms and Depo-Provera". Contraception. 57 (4): 237–40. doi:10.1016/s0010-7824(98)00024-9. PMID 9649914.
- ^ Kahn LS, Halbreich U (September 2001). "Oral contraceptives and mood". Expert Opin Pharmacother. 2 (9): 1367–82. doi:10.1517/14656566.2.9.1367. PMID 11585017. S2CID 45061663.
- ^ Lanza di Scalea T, Pearlstein T (July 2019). "Premenstrual Dysphoric Disorder". The Medical Clinics of North America. 103 (4): 613–628. doi:10.1016/j.mcna.2019.02.007. PMID 31078196. S2CID 153307984.
- ^ Ma S, Song SJ (June 2023). "Oral contraceptives containing drospirenone for premenstrual syndrome". The Cochrane Database of Systematic Reviews. 2023 (6): CD006586. doi:10.1002/14651858.CD006586.pub5. PMC 10289136. PMID 37365881.
- ^ a b c Regidor PA, Schindler AE (October 2017). "Antiandrogenic and antimineralocorticoid health benefits of COC containing newer progestogens: dienogest and drospirenone". Oncotarget. 8 (47): 83334–83342. doi:10.18632/oncotarget.19833. PMC 5669973. PMID 29137347.
- ^ a b c Lewis CA, Kimmig AS, Zsido RG, Jank A, Derntl B, Sacher J (November 2019). "Effects of Hormonal Contraceptives on Mood: A Focus on Emotion Recognition and Reactivity, Reward Processing, and Stress Response". Current Psychiatry Reports. 21 (11): 115. doi:10.1007/s11920-019-1095-z. PMC 6838021. PMID 31701260.
- ^ Pagano HP, Zapata LB, Berry-Bibee EN, Nanda K, Curtis KM (December 2016). "Safety of hormonal contraception and intrauterine devices among women with depressive and bipolar disorders: a systematic review". Contraception. 94 (6): 641–649. doi:10.1016/j.contraception.2016.06.012. PMC 10994544. PMID 27364100.
- ^ Dennis CL, Ross LE, Herxheimer A (October 2008). "Oestrogens and progestins for preventing and treating postpartum depression". The Cochrane Database of Systematic Reviews. 2008 (4): CD001690. doi:10.1002/14651858.CD001690.pub2. PMC 7061327. PMID 18843619.
- ^ a b Maki PM, Kornstein SG, Joffe H, Bromberger JT, Freeman EW, Athappilly G, Bobo WV, Rubin LH, Koleva HK, Cohen LS, Soares CN (February 2019). "Guidelines for the Evaluation and Treatment of Perimenopausal Depression: Summary and Recommendations". J Womens Health (Larchmt). 28 (2): 117–134. doi:10.1089/jwh.2018.27099.mensocrec. PMID 30182804.
- ^ a b Stute P, Spyropoulou A, Karageorgiou V, Cano A, Bitzer J, Ceausu I, Chedraui P, Durmusoglu F, Erkkola R, Goulis DG, Lindén Hirschberg A, Kiesel L, Lopes P, Pines A, Rees M, van Trotsenburg M, Zervas I, Lambrinoudaki I (January 2020). "Management of depressive symptoms in peri- and postmenopausal women: EMAS position statement". Maturitas. 131: 91–101. doi:10.1016/j.maturitas.2019.11.002. PMID 31740049.
- ^ Gava G, Orsili I, Alvisi S, Mancini I, Seracchioli R, Meriggiola MC (October 2019). "Cognition, Mood and Sleep in Menopausal Transition: The Role of Menopause Hormone Therapy". Medicina. 55 (10): 668. doi:10.3390/medicina55100668. PMC 6843314. PMID 31581598.
- ^ Toffol E, Heikinheimo O, Partonen T (May 2015). "Hormone therapy and mood in perimenopausal and postmenopausal women: a narrative review". Menopause. 22 (5): 564–78. doi:10.1097/GME.0000000000000323. PMID 25203891. S2CID 5830652.
- ^ Zweifel JE, O'Brien WH (April 1997). "A meta-analysis of the effect of hormone replacement therapy upon depressed mood". Psychoneuroendocrinology. 22 (3): 189–212. doi:10.1016/s0306-4530(96)00034-0. PMID 9203229. S2CID 44630030.
- ^ Rogerio A. Lobo (5 June 2007). Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. Elsevier. pp. 211–. ISBN 978-0-08-055309-2.
- ^ Gordon JL, Girdler SS (December 2014). "Hormone replacement therapy in the treatment of perimenopausal depression". Curr Psychiatry Rep. 16 (12): 517. doi:10.1007/s11920-014-0517-1. PMID 25308388. S2CID 23794180.
- ^ Fischer B, Gleason C, Asthana S (April 2014). "Effects of hormone therapy on cognition and mood". Fertil. Steril. 101 (4): 898–904. doi:10.1016/j.fertnstert.2014.02.025. PMC 4330961. PMID 24680649.
- ^ Prior JC (August 2018). "Progesterone for treatment of symptomatic menopausal women". Climacteric. 21 (4): 358–365. doi:10.1080/13697137.2018.1472567. PMID 29962247.
- ^ a b Pastor Z, Holla K, Chmel R (February 2013). "The influence of combined oral contraceptives on female sexual desire: a systematic review". Eur J Contracept Reprod Health Care. 18 (1): 27–43. doi:10.3109/13625187.2012.728643. PMID 23320933. S2CID 34748865.
- ^ Zimmerman Y, Eijkemans MJ, Coelingh Bennink HJ, Blankenstein MA, Fauser BC (2014). "The effect of combined oral contraception on testosterone levels in healthy women: a systematic review and meta-analysis". Hum. Reprod. Update. 20 (1): 76–105. doi:10.1093/humupd/dmt038. PMC 3845679. PMID 24082040.
- ^ Casado-Espada NM, de Alarcón R, de la Iglesia-Larrad JI, Bote-Bonaechea B, Montejo ÁL (June 2019). "Hormonal Contraceptives, Female Sexual Dysfunction, and Managing Strategies: A Review". Journal of Clinical Medicine. 8 (6): 908. doi:10.3390/jcm8060908. PMC 6617135. PMID 31242625.
- ^ a b c "Deep Vein Thrombosis". NHLBI, NIH. Retrieved 28 December 2019.
- ^ a b c d e Sitruk-Ware R, Nath A (February 2013). "Characteristics and metabolic effects of estrogen and progestins contained in oral contraceptive pills". Best Practice & Research. Clinical Endocrinology & Metabolism. 27 (1): 13–24. doi:10.1016/j.beem.2012.09.004. PMID 23384742.
- ^ a b c d e f g h Pfeifer S, Butts S, Dumesic D, Fossum G, Gracia C, La Barbera A, Mersereau J, Odem R, Penzias A, Pisarska M, Rebar R, Reindollar R, Rosen M, Sandlow J, Sokol R, Vernon M, Widra E (January 2017). "Combined hormonal contraception and the risk of venous thromboembolism: a guideline". Fertility and Sterility. 107 (1): 43–51. doi:10.1016/j.fertnstert.2016.09.027. PMID 27793376.
- ^ a b Skouby SO, Sidelmann JJ (November 2018). "Impact of progestogens on hemostasis". Hormone Molecular Biology and Clinical Investigation. 37 (2). doi:10.1515/hmbci-2018-0041. PMID 30447140. S2CID 53875910.
- ^ Barco S, Nijkeuter M, Middeldorp S (July 2013). "Pregnancy and venous thromboembolism". Seminars in Thrombosis and Hemostasis. 39 (5): 549–558. doi:10.1055/s-0033-1343893. PMID 23633191. S2CID 5521763.
- ^ Simon T, Beau Yon de Jonage-Canonico M, Oger E, Wahl D, Conard J, Meyer G, Emmerich J, Barrellier MT, Guiraud A, Scarabin PY (January 2006). "Indicators of lifetime endogenous estrogen exposure and risk of venous thromboembolism". Journal of Thrombosis and Haemostasis. 4 (1): 71–76. doi:10.1111/j.1538-7836.2005.01693.x. PMID 16409454. S2CID 24161765.
- ^ Canonico M, Plu-Bureau G, O'Sullivan MJ, Stefanick ML, Cochrane B, Scarabin PY, Manson JE (March 2014). "Age at menopause, reproductive history, and venous thromboembolism risk among postmenopausal women: the Women's Health Initiative Hormone Therapy clinical trials". Menopause. 21 (3): 214–220. doi:10.1097/GME.0b013e31829752e0. PMC 3815514. PMID 23760439.
- ^ a b c d e f g h i j k Sitruk-Ware R, Nath A (June 2011). "Metabolic effects of contraceptive steroids". Reviews in Endocrine & Metabolic Disorders. 12 (2): 63–75. doi:10.1007/s11154-011-9182-4. PMID 21538049. S2CID 23760705.
- ^ a b c d Schindler AE (December 2003). "Differential effects of progestins on hemostasis". Maturitas. 46 (Suppl 1): S31–7. doi:10.1016/j.maturitas.2003.09.016. PMID 14670643.
- ^ a b c Wiegratz I, Kuhl H (September 2006). "Metabolic and clinical effects of progestogens". The European Journal of Contraception & Reproductive Health Care. 11 (3): 153–161. doi:10.1080/13625180600772741. PMID 17056444. S2CID 27088428.
- ^ a b Kuhl H (May 1996). "Effects of progestogens on haemostasis". Maturitas. 24 (1–2): 1–19. doi:10.1016/0378-5122(96)00994-2. PMID 8794429.
- ^ Tepper NK, Whiteman MK, Marchbanks PA, James AH, Curtis KM (December 2016). "Progestin-only contraception and thromboembolism: A systematic review". Contraception. 94 (6): 678–700. doi:10.1016/j.contraception.2016.04.014. PMC 11034842. PMID 27153743.
- ^ Mantha S, Karp R, Raghavan V, Terrin N, Bauer KA, Zwicker JI (August 2012). "Assessing the risk of venous thromboembolic events in women taking progestin-only contraception: a meta-analysis". BMJ. 345: e4944. doi:10.1136/bmj.e4944. PMC 3413580. PMID 22872710.
- ^ a b c Blanco-Molina MA, Lozano M, Cano A, Cristobal I, Pallardo LP, Lete I (May 2012). "Progestin-only contraception and venous thromboembolism". Thrombosis Research. 129 (5): e257 – e262. doi:10.1016/j.thromres.2012.02.042. PMID 22425318. S2CID 261804433.
- ^ a b c Rott H (February 2019). "Birth Control Pills and Thrombotic Risks: Differences of Contraception Methods with and without Estrogen". Hamostaseologie. 39 (1): 42–48. doi:10.1055/s-0039-1677806. PMID 30669160. S2CID 58947063.
- ^ a b c d e Beyer-Westendorf J, Bauersachs R, Hach-Wunderle V, Zotz RB, Rott H (October 2018). "Sex hormones and venous thromboembolism - from contraception to hormone replacement therapy". VASA. Zeitschrift für Gefässkrankheiten. 47 (6): 441–450. doi:10.1024/0301-1526/a000726. PMID 30008249. S2CID 51628832.
- ^ a b DeLoughery TG (June 2011). "Estrogen and thrombosis: controversies and common sense". Reviews in Endocrine & Metabolic Disorders. 12 (2): 77–84. doi:10.1007/s11154-011-9178-0. PMID 21559819. S2CID 28053690.
- ^ Mantha S, Karp R, Raghavan V, Terrin N, Bauer KA, Zwicker JI (August 2012). "Assessing the risk of venous thromboembolic events in women taking progestin-only contraception: a meta-analysis". BMJ. 345 (aug07 2): e4944. doi:10.1136/bmj.e4944. PMC 3413580. PMID 22872710.
- ^ a b c d e Scarabin PY (August 2018). "Progestogens and venous thromboembolism in menopausal women: an updated oral versus transdermal estrogen meta-analysis". Climacteric. 21 (4): 341–345. doi:10.1080/13697137.2018.1446931. PMID 29570359. S2CID 4229701.
- ^ Tepper NK, Jeng G, Curtis KM, Boutot ME, Boulet SL, Whiteman MK (March 2019). "Venous Thromboembolism Among Women Initiating Depot Medroxyprogesterone Acetate Immediately Postpartum". Obstetrics and Gynecology. 133 (3): 533–540. doi:10.1097/AOG.0000000000003135. PMC 10983016. PMID 30741807.
- ^ a b c d e Gourdy P, Bachelot A, Catteau-Jonard S, Chabbert-Buffet N, Christin-Maître S, Conard J, Fredenrich A, Gompel A, Lamiche-Lorenzini F, Moreau C, Plu-Bureau G, Vambergue A, Vergès B, Kerlan V (November 2012). "Hormonal contraception in women at risk of vascular and metabolic disorders: guidelines of the French Society of Endocrinology". Annales d'Endocrinologie. 73 (5): 469–487. doi:10.1016/j.ando.2012.09.001. PMID 23078975.
- ^ Conard J, Plu-Bureau G, Bahi N, Horellou MH, Pelissier C, Thalabard JC (December 2004). "Progestogen-only contraception in women at high risk of venous thromboembolism". Contraception. 70 (6): 437–441. doi:10.1016/j.contraception.2004.07.009. PMID 15541404.
- ^ a b Beyer-Westendorf J, Werth S, Halbritter K, Weiss N (April 2010). "Cancer in males and risk of venous thromboembolism". Thromb. Res. 125 (Suppl 2): S155–9. doi:10.1016/S0049-3848(10)70035-9. PMID 20433997.
- ^ Guay DR (December 2008). "Inappropriate sexual behaviors in cognitively impaired older individuals". Am J Geriatr Pharmacother. 6 (5): 269–88. doi:10.1016/j.amjopharm.2008.12.004. PMID 19161930.
- ^ a b Seaman HE, Langley SE, Farmer RD, de Vries CS (June 2007). "Venous thromboembolism and cyproterone acetate in men with prostate cancer: a study using the General Practice Research Database". BJU Int. 99 (6): 1398–403. doi:10.1111/j.1464-410X.2007.06859.x. PMID 17537215. S2CID 21350686.
- ^ a b Van Hemelrijck M, Adolfsson J, Garmo H, Bill-Axelson A, Bratt O, Ingelsson E, Lambe M, Stattin P, Holmberg L (May 2010). "Risk of thromboembolic diseases in men with prostate cancer: results from the population-based PCBaSe Sweden". Lancet Oncol. 11 (5): 450–8. doi:10.1016/S1470-2045(10)70038-3. PMC 2861771. PMID 20395174.
- ^ Schröder FH, Radlmaier A (2009). "Steroidal Antiandrogens". In Jordan VC, Furr BJ (eds.). Hormone Therapy in Breast and Prostate Cancer. Humana Press. pp. 325–346. doi:10.1007/978-1-59259-152-7_15. ISBN 978-1-60761-471-5.
- ^ Namer M (October 1988). "Clinical applications of antiandrogens". J. Steroid Biochem. 31 (4B): 719–29. doi:10.1016/0022-4731(88)90023-4. PMID 2462132.
- ^ a b c d e Asscheman H, T'Sjoen G, Lemaire A, Mas M, Meriggiola MC, Mueller A, Kuhn A, Dhejne C, Morel-Journel N, Gooren LJ (September 2014). "Venous thrombo-embolism as a complication of cross-sex hormone treatment of male-to-female transsexual subjects: a review". Andrologia. 46 (7): 791–5. doi:10.1111/and.12150. hdl:11585/413984. PMID 23944849. S2CID 5363824.
- ^ a b c d Rovinski D, Ramos RB, Fighera TM, Casanova GK, Spritzer PM (August 2018). "Risk of venous thromboembolism events in postmenopausal women using oral versus non-oral hormone therapy: A systematic review and meta-analysis". Thromb. Res. 168: 83–95. doi:10.1016/j.thromres.2018.06.014. PMID 29936403. S2CID 49421543.
- ^ a b c d e Han L, Jensen JT (December 2015). "Does the Progestogen Used in Combined Hormonal Contraception Affect Venous Thrombosis Risk?". Obstet. Gynecol. Clin. North Am. 42 (4): 683–98. doi:10.1016/j.ogc.2015.07.007. PMID 26598309.
- ^ a b c d Bateson D, Butcher BE, Donovan C, Farrell L, Kovacs G, Mezzini T, Raynes-Greenow C, Pecoraro G, Read C, Baber R (2016). "Risk of venous thromboembolism in women taking the combined oral contraceptive: A systematic review and meta-analysis". Aust Fam Physician. 45 (1): 59–64. PMID 27051991.
- ^ a b c d e Vinogradova Y, Coupland C, Hippisley-Cox J (January 2019). "Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases". BMJ. 364: k4810. doi:10.1136/bmj.k4810. PMC 6326068. PMID 30626577.
- ^ a b c d Vinogradova Y, Coupland C, Hippisley-Cox J (May 2015). "Use of combined oral contraceptives and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases". BMJ. 350: h2135. doi:10.1136/bmj.h2135. PMC 4444976. PMID 26013557.
- ^ a b c Plu-Bureau G, Maitrot-Mantelet L, Hugon-Rodin J, Canonico M (February 2013). "Hormonal contraceptives and venous thromboembolism: an epidemiological update". Best Pract. Res. Clin. Endocrinol. Metab. 27 (1): 25–34. doi:10.1016/j.beem.2012.11.002. PMID 23384743.
- ^ a b Connors JM, Middeldorp S (November 2019). "Transgender patients and the role of the coagulation clinician". J. Thromb. Haemost. 17 (11): 1790–1797. doi:10.1111/jth.14626. PMID 31465627. S2CID 201673648.
- ^ Oedingen C, Scholz S, Razum O (May 2018). "Systematic review and meta-analysis of the association of combined oral contraceptives on the risk of venous thromboembolism: The role of the progestogen type and estrogen dose". Thromb. Res. 165: 68–78. doi:10.1016/j.thromres.2018.03.005. PMID 29573722.
- ^ Dragoman MV, Tepper NK, Fu R, Curtis KM, Chou R, Gaffield ME (June 2018). "A systematic review and meta-analysis of venous thrombosis risk among users of combined oral contraception". Int J Gynaecol Obstet. 141 (3): 287–294. doi:10.1002/ijgo.12455. PMC 5969307. PMID 29388678.
- ^ Batur P, Casey PM (February 2017). "Drospirenone Litigation: Does the Punishment Fit the Crime?". J Womens Health (Larchmt). 26 (2): 99–102. doi:10.1089/jwh.2016.6092. PMID 27854556.
- ^ a b c Sitruk-Ware R (November 2016). "Hormonal contraception and thrombosis". Fertil. Steril. 106 (6): 1289–1294. doi:10.1016/j.fertnstert.2016.08.039. PMID 27678035.
- ^ a b Nelson AL (2015). "An update on new orally administered contraceptives for women". Expert Opin Pharmacother. 16 (18): 2759–72. doi:10.1517/14656566.2015.1100173. PMID 26512437. S2CID 207481206.
- ^ Farris M, Bastianelli C, Rosato E, Brosens I, Benagiano G (October 2017). "Pharmacodynamics of combined estrogen-progestin oral contraceptives: 2. effects on hemostasis". Expert Rev Clin Pharmacol. 10 (10): 1129–1144. doi:10.1080/17512433.2017.1356718. PMID 28712325. S2CID 205931204.
- ^ a b Fruzzetti F, Cagnacci A (2018). "Venous thrombosis and hormonal contraception: what's new with estradiol-based hormonal contraceptives?". Open Access J Contracept. 9: 75–79. doi:10.2147/OAJC.S179673. PMC 6239102. PMID 30519125.
- ^ a b Grandi G, Facchinetti F, Bitzer J (August 2017). "Estradiol in hormonal contraception: real evolution or just same old wine in a new bottle?". Eur J Contracept Reprod Health Care. 22 (4): 245–246. doi:10.1080/13625187.2017.1372571. hdl:11380/1153791. PMID 28902531.
- ^ a b c d e f g h i j k l m Stanczyk FZ, Hapgood JP, Winer S, Mishell DR (April 2013). "Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects". Endocrine Reviews. 34 (2): 171–208. doi:10.1210/er.2012-1008. PMC 3610676. PMID 23238854.
- ^ a b c Canonico M, Plu-Bureau G, Scarabin PY (December 2011). "Progestogens and venous thromboembolism among postmenopausal women using hormone therapy" (PDF). Maturitas. 70 (4): 354–60. doi:10.1016/j.maturitas.2011.10.002. PMID 22024394.
- ^ Stevenson JC, Panay N, Pexman-Fieth C (September 2013). "Oral estradiol and dydrogesterone combination therapy in postmenopausal women: review of efficacy and safety". Maturitas. 76 (1): 10–21. doi:10.1016/j.maturitas.2013.05.018. PMID 23835005.
Dydrogesterone did not increase the risk of VTE associated with oral estrogen (odds ratio (OR) 0.9, 95% CI 0.4–2.3). Other progestogens (OR 3.9, 95% CI 1.5–10.0) were found to further increase the risk of VTE associated with oral estrogen (OR 4.2, 95% CI 1.5–11.6).
- ^ Schneider C, Jick SS, Meier CR (October 2009). "Risk of cardiovascular outcomes in users of estradiol/dydrogesterone or other HRT preparations". Climacteric. 12 (5): 445–53. doi:10.1080/13697130902780853. PMID 19565370. S2CID 45890629.
- ^ a b c d e f g h i Davey DA (March 2018). "Menopausal hormone therapy: a better and safer future". Climacteric. 21 (5): 454–461. doi:10.1080/13697137.2018.1439915. PMID 29526116. S2CID 3850275.
- ^ a b c d e Goldstein Z, Khan M, Reisman T, Safer JD (2019). "Managing the risk of venous thromboembolism in transgender adults undergoing hormone therapy". J Blood Med. 10: 209–216. doi:10.2147/JBM.S166780. PMC 6628137. PMID 31372078.
- ^ Roach RE, Lijfering WM, Helmerhorst FM, Cannegieter SC, Rosendaal FR, van Hylckama Vlieg A (January 2013). "The risk of venous thrombosis in women over 50 years old using oral contraception or postmenopausal hormone therapy". J. Thromb. Haemost. 11 (1): 124–31. doi:10.1111/jth.12060. PMID 23136837. S2CID 22306721.
- ^ a b c Odlind V, Milsom I, Persson I, Victor A (June 2002). "Can changes in sex hormone binding globulin predict the risk of venous thromboembolism with combined oral contraceptive pills?". Acta Obstet Gynecol Scand. 81 (6): 482–90. doi:10.1034/j.1600-0412.2002.810603.x. PMID 12047300. S2CID 26054257.
- ^ Raps M, Helmerhorst F, Fleischer K, Thomassen S, Rosendaal F, Rosing J, Ballieux B, VAN Vliet H (June 2012). "Sex hormone-binding globulin as a marker for the thrombotic risk of hormonal contraceptives". J. Thromb. Haemost. 10 (6): 992–7. doi:10.1111/j.1538-7836.2012.04720.x. PMID 22469296. S2CID 20803995.
- ^ Christin-Maitre S (2016). "Risque cardiovasculaire de la contraception hormonale chez la femme" [Cardiovacular risk of hormonal contraception in women]. Bulletin de l'Académie Nationale de Médecine. 200 (7): 1485–1496. doi:10.1016/S0001-4079(19)30619-3. ISSN 0001-4079.
- ^ Stephen J. Winters, Ilpo T. Huhtaniemi (25 April 2017). Male Hypogonadism: Basic, Clinical and Therapeutic Principles. Humana Press. pp. 307–. ISBN 978-3-319-53298-1.
- ^ Notelovitz M (March 2006). "Clinical opinion: the biologic and pharmacologic principles of estrogen therapy for symptomatic menopause". MedGenMed. 8 (1): 85. PMC 1682006. PMID 16915215.
- ^ Goodman MP (February 2012). "Are all estrogens created equal? A review of oral vs. transdermal therapy". J Womens Health (Larchmt). 21 (2): 161–9. doi:10.1089/jwh.2011.2839. PMID 22011208.
- ^ a b Stege R, Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A (1988). "Single drug polyestradiol phosphate therapy in prostatic cancer". Am. J. Clin. Oncol. 11 (Suppl 2): S101–3. doi:10.1097/00000421-198801102-00024. PMID 3242384. S2CID 32650111.
- ^ a b von Schoultz B, Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A, Stege R (1989). "Estrogen therapy and liver function--metabolic effects of oral and parenteral administration". Prostate. 14 (4): 389–95. doi:10.1002/pros.2990140410. PMID 2664738. S2CID 21510744.
- ^ Ottosson UB, Carlström K, Johansson BG, von Schoultz B (1986). "Estrogen induction of liver proteins and high-density lipoprotein cholesterol: comparison between estradiol valerate and ethinyl estradiol". Gynecol. Obstet. Invest. 22 (4): 198–205. doi:10.1159/000298914. PMID 3817605.
- ^ Fruzzetti F, Trémollieres F, Bitzer J (May 2012). "An overview of the development of combined oral contraceptives containing estradiol: focus on estradiol valerate/dienogest". Gynecol. Endocrinol. 28 (5): 400–8. doi:10.3109/09513590.2012.662547. PMC 3399636. PMID 22468839.
- ^ Tangpricha V, den Heijer M (April 2017). "Oestrogen and anti-androgen therapy for transgender women". The Lancet. Diabetes & Endocrinology. 5 (4): 291–300. doi:10.1016/S2213-8587(16)30319-9. PMC 5366074. PMID 27916515.
- ^ Weinand JD, Safer JD (June 2015). "Hormone therapy in transgender adults is safe with provider supervision; A review of hormone therapy sequelae for transgender individuals". Journal of Clinical & Translational Endocrinology. 2 (2): 55–60. doi:10.1016/j.jcte.2015.02.003. PMC 5226129. PMID 28090436.
- ^ Price S, McManus J, Barrett J (2019). "The transgender population: improving awareness for gynaecologists and their role in the provision of care". The Obstetrician & Gynaecologist. 21 (1): 11–20. doi:10.1111/tog.12521. ISSN 1467-2561.
- ^ Asscheman H, Gooren LJ (1993). "Hormone Treatment in Transsexuals". Journal of Psychology & Human Sexuality. 5 (4): 39–54. doi:10.1300/J056v05n04_03. ISSN 0890-7064. S2CID 144580633.
- ^ Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T'Sjoen GG (December 2017). "Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline". Endocrine Practice. 23 (12): 1437. doi:10.4158/1934-2403-23.12.1437. PMID 29320642. S2CID 3639218.
- ^ Prentice RL, Anderson GL (2008). "The women's health initiative: lessons learned". Annual Review of Public Health. 29: 131–150. doi:10.1146/annurev.publhealth.29.020907.090947. PMID 18348708.
- ^ Prentice RL (November 2014). "Postmenopausal hormone therapy and the risks of coronary heart disease, breast cancer, and stroke". Seminars in Reproductive Medicine. 32 (6): 419–425. doi:10.1055/s-0034-1384624. PMC 4212810. PMID 25321418.
- ^ Bassuk SS, Manson JE (2008). "Women's Health Initiative Hormone Therapy Trials". Wiley Encyclopedia of Clinical Trials. pp. 1–10. doi:10.1002/9780471462422.eoct391. ISBN 978-0-471-46242-2.
- ^ a b c d e Hermsmeyer RK, Thompson TL, Pohost GM, Kaski JC (July 2008). "Cardiovascular effects of medroxyprogesterone acetate and progesterone: a case of mistaken identity?". Nature Clinical Practice. Cardiovascular Medicine. 5 (7): 387–395. doi:10.1038/ncpcardio1234. PMID 18521110. S2CID 39945411.
- ^ Sitruk-Ware R, El-Etr M (August 2013). "Progesterone and related progestins: potential new health benefits". Climacteric. 16 (Suppl 1): 69–78. doi:10.3109/13697137.2013.802556. PMID 23647429. S2CID 25447915.
- ^ Nath A, Sitruk-Ware R (2009). "Different cardiovascular effects of progestins according to structure and activity". Climacteric. 12 (Suppl 1): 96–101. doi:10.1080/13697130902905757. PMID 19811251. S2CID 2987558.
- ^ Sitruk-Ware R (October 2005). "Pharmacology of different progestogens: the special case of drospirenone". Climacteric. 8 (Suppl 3): 4–12. doi:10.1080/13697130500330382. PMID 16203650. S2CID 24205704.
- ^ Sitruk-Ware RL (October 2003). "Hormone therapy and the cardiovascular system: the critical role of progestins". Climacteric. 6 (Suppl 3): 21–28. PMID 15018245.
- ^ Boardman HM, Hartley L, Eisinga A, Main C, Roqué i Figuls M, Bonfill Cosp X, Gabriel Sanchez R, Knight B (March 2015). "Hormone therapy for preventing cardiovascular disease in post-menopausal women". The Cochrane Database of Systematic Reviews. 2015 (3): CD002229. doi:10.1002/14651858.CD002229.pub4. hdl:20.500.12105/9999. PMC 10183715. PMID 25754617.
- ^ a b Jiang Y, Tian W (November 2017). "The effects of progesterones on blood lipids in hormone replacement therapy". Lipids in Health and Disease. 16 (1): 219. doi:10.1186/s12944-017-0612-5. PMC 5697110. PMID 29157280.
- ^ Nath A, Sitruk-Ware R (April 2009). "Parenteral administration of progestins for hormonal replacement therapy". Eur J Contracept Reprod Health Care. 14 (2): 88–96. doi:10.1080/13625180902747425. PMID 19340703. S2CID 43025098.
- ^ a b c d e f g h i Collaborative Group on Hormonal Factors in Breast Cancer (September 2019). "Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence". Lancet. 394 (10204): 1159–1168. doi:10.1016/S0140-6736(19)31709-X. PMC 6891893. PMID 31474332.
- ^ a b c d e f Yang Z, Hu Y, Zhang J, Xu L, Zeng R, Kang D (2017). "Estradiol therapy and breast cancer risk in perimenopausal and postmenopausal women: a systematic review and meta-analysis". Gynecol. Endocrinol. 33 (2): 87–92. doi:10.1080/09513590.2016.1248932. PMID 27898258. S2CID 205631264.
- ^ a b Lambrinoudaki I (2014). "Progestogens in postmenopausal hormone therapy and the risk of breast cancer". Maturitas. 77 (4): 311–7. doi:10.1016/j.maturitas.2014.01.001. PMID 24485796.
- ^ Beral V, Peto R, Pirie K, Reeves G (September 2019). "Menopausal hormone therapy and 20-year breast cancer mortality". Lancet. 394 (10204): 1139. doi:10.1016/S0140-6736(19)32033-1. PMID 31474331.
- ^ Stanczyk FZ, Bhavnani BR (July 2014). "Use of medroxyprogesterone acetate for hormone therapy in postmenopausal women: is it safe?". J. Steroid Biochem. Mol. Biol. 142: 30–8. doi:10.1016/j.jsbmb.2013.11.011. PMID 24291402. S2CID 22731802.
- ^ a b c Sturdee DW (August 2013). "Are progestins really necessary as part of a combined HRT regimen?". Climacteric. 16 (Suppl 1): 79–84. doi:10.3109/13697137.2013.803311. PMID 23651281. S2CID 21894200.
- ^ Mirkin S (August 2018). "Evidence on the use of progesterone in menopausal hormone therapy". Climacteric. 21 (4): 346–354. doi:10.1080/13697137.2018.1455657. PMID 29630427.
- ^ a b c d Kuhl H, Schneider HP (August 2013). "Progesterone – promoter or inhibitor of breast cancer". Climacteric. 16 (Suppl 1): 54–68. doi:10.3109/13697137.2013.768806. PMID 23336704. S2CID 20808536.
- ^ a b c de Blok CJ, Wiepjes CM, Nota NM, van Engelen K, Adank MA, Dreijerink KM, Barbé E, Konings IR, den Heijer M (May 2019). "Breast cancer risk in transgender people receiving hormone treatment: nationwide cohort study in the Netherlands". BMJ. 365: l1652. doi:10.1136/bmj.l1652. PMC 6515308. PMID 31088823.
- ^ a b c de Blok CJ, Dreijerink KM, den Heijer M (June 2019). "Cancer Risk in Transgender People". Endocrinol. Metab. Clin. North Am. 48 (2): 441–452. doi:10.1016/j.ecl.2019.02.005. PMID 31027551. S2CID 135382400.
- ^ a b c Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, Wilson DP, Nota NM, den Heijer M, Gooren LJ (2000). "Evaluation and Treatment of Gender-Dysphoric/Gender Incongruent Adults". Endotext [Internet]. PMID 31343858.
- ^ a b c Iwamoto SJ, Defreyne J, Rothman MS, Van Schuylenbergh J, Van de Bruaene L, Motmans J, T'Sjoen G (2019). "Health considerations for transgender women and remaining unknowns: a narrative review". Ther Adv Endocrinol Metab. 10: 2042018819871166. doi:10.1177/2042018819871166. PMC 6719479. PMID 31516689.
- ^ Jacobsen BM, Horwitz KB (2012). "Progesterone receptors, their isoforms and progesterone regulated transcription". Mol. Cell. Endocrinol. 357 (1–2): 18–29. doi:10.1016/j.mce.2011.09.016. PMC 3272316. PMID 21952082.
- ^ Scarpin KM, Graham JD, Mote PA, Clarke CL (2009). "Progesterone action in human tissues: regulation by progesterone receptor (PR) isoform expression, nuclear positioning and coregulator expression". Nucl Recept Signal. 7: e009. doi:10.1621/nrs.07009. PMC 2807635. PMID 20087430.
- ^ Thomas P, Pang Y (2012). "Membrane progesterone receptors: evidence for neuroprotective, neurosteroid signaling and neuroendocrine functions in neuronal cells". Neuroendocrinology. 96 (2): 162–71. doi:10.1159/000339822. PMC 3489003. PMID 22687885.
- ^ Petersen SL, Intlekofer KA, Moura-Conlon PJ, Brewer DN, Del Pino Sans J, Lopez JA (2013). "Novel progesterone receptors: neural localization and possible functions". Frontiers in Neuroscience. 7: 164. doi:10.3389/fnins.2013.00164. PMC 3776953. PMID 24065878.
- ^ Gompel A, Plu-Bureau G (August 2018). "Progesterone, progestins and the breast in menopause treatment". Climacteric. 21 (4): 326–332. doi:10.1080/13697137.2018.1476483. PMID 29852797. S2CID 46922084.
- ^ Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH (December 2003). "Classification and pharmacology of progestins". Maturitas. 46 Suppl 1: S7 – S16. doi:10.1016/j.maturitas.2003.09.014. PMID 14670641.
- ^ Kuhl H (2011). "Pharmacology of Progestogens" (PDF). J Reproduktionsmed Endokrinol. 8 (1): 157–177.
- ^ Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- ^ Lauritzen C (September 1990). "Clinical use of oestrogens and progestogens". Maturitas. 12 (3): 199–214. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- ^ Kuhl H (September 1990). "Pharmacokinetics of oestrogens and progestogens". Maturitas. 12 (3): 171–97. doi:10.1016/0378-5122(90)90003-o. PMID 2170822.
- ^ Knörr K, Knörr-Gärtner H, Beller FK, Lauritzen C (8 March 2013). Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion. Springer-Verlag. pp. 583–. ISBN 978-3-642-95583-9.
- ^ Knörr K, Beller FK, Lauritzen C (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. pp. 214–. ISBN 978-3-662-00942-0.
- ^ Horský J, Presl J (1981). "Hormonal Treatment of Disorders of the Menstrual Cycle". In J. Horsky, J. Presl (eds.). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 309–332. doi:10.1007/978-94-009-8195-9_11. ISBN 978-94-009-8195-9.
- ^ Ferin J (September 1972). "Orally Active Progestational Compounds. Human Studies: Effects on the Utero-Vaginal Tract". In M. Tausk (ed.). Pharmacology of the Endocrine System and Related Drugs: Progesterone, Progestational Drugs and Antifertility Agents. Vol. II. Pergamon Press. pp. 245–273. ISBN 978-0080168128. OCLC 278011135.
- ^ Freimut A. Leidenberger, Thomas Strowitzki, Olaf Ortmann (29 August 2009). Klinische Endokrinologie für Frauenärzte. Springer-Verlag. pp. 225, 227. ISBN 978-3-540-89760-6.
- ^ Neumann F, Düsterberg B (1998). "Entwicklung auf dem Gebiet der Gestagene" [Development in the field of progestogens]. Reproduktionsmedizin. 14 (4): 257–264. doi:10.1007/s004440050042. ISSN 1434-6931.
- ^ Hammerstein J (1990). "Antiandrogens: Clinical Aspects". Hair and Hair Diseases. pp. 827–886. doi:10.1007/978-3-642-74612-3_35.
- ^ Willibald Pschyrembel (1968). Praktische Gynäkologie: für Studierende und Ärzte. Walter de Gruyter. p. 599. ISBN 978-3-11-150424-7.
- ^ Ufer J (1968). "Die therapeutische Anwendung der Gestagene beim Menschen" [Therapeutic Use of Progestagens in Humans]. Die Gestagene [Progestogens]. Springer-Verlag. pp. 1026–1124. doi:10.1007/978-3-642-99941-3_7. ISBN 978-3-642-99941-3.
Zur Transformation des Endometriums benotigten sie 200-400 mg [ethisterone] pro Cyclus und postulierten eine etwa sechsfach schwachere Wirkung gegenuber dem Progesteron i.m. appliziert.
- ^ a b Endrikat J, Gerlinger C, Richard S, Rosenbaum P, Düsterberg B (December 2011). "Ovulation inhibition doses of progestins: a systematic review of the available literature and of marketed preparations worldwide". Contraception. 84 (6): 549–57. doi:10.1016/j.contraception.2011.04.009. PMID 22078182.
Table 1 Publications on ovulation inhibition doses of progestins: Progestin: Progesterone. Reference: Pincus (1956). Method: Urinary Pdiol. Daily dose (mg): 300.000. Total number of cycles in all subjects: 61. Total number of ovulation in all subjects: 30. % of ovulation in all subjects: 49.
- ^ Milan Rastislav Henzl, John A. Edwards (10 November 1999). "Pharmacology of Progestins: 17α-Hydroxyprogesterone Derivatives and Progestins of the First and Second Generation". In Régine Sitruk-Ware, Daniel R. Mishell (eds.). Progestins and Antiprogestins in Clinical Practice. Taylor & Francis. pp. 101–132. ISBN 978-0-8247-8291-7.
- ^ Kopera H (1991). "Hormone der Gonaden". Hormonelle Therapie für die Frau. pp. 59–124. doi:10.1007/978-3-642-95670-6_6. ISBN 978-3-642-95670-6. ISSN 0172-777X.
- ^ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, World Health Organization, International Agency for Research on Cancer (2007). "Annex 2: Composition of Oral and Injectable Estrogen–Progestogen Contraceptives". Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 431–464. ISBN 978-92-832-1291-1.
- ^ Lobo RA, Stanczyk FZ (1994). "New knowledge in the physiology of hormonal contraceptives". American Journal of Obstetrics and Gynecology. 170 (5): 1499–1507. doi:10.1016/S0002-9378(12)91807-4. ISSN 0002-9378.
- ^ Henzl MR (1986). "Contraceptive Hormones and their Clinical Use". In Samuel S. C. Yen, Robert B. Jaffe (eds.). Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Saunders. pp. 643–682. ISBN 978-0-7216-9630-0.
- ^ Ostergaard E (February 1965). "The oral progestational and anti-ovulatory properties of megestrol acetate and its therapeutic use in gynaecological disorders". J Obstet Gynaecol Br Emp. 72 (1): 45–48. doi:10.1111/j.1471-0528.1965.tb01372.x. PMID 12332461.
The anti-ovulatory properties of megestrol acetate 5 mg. plus Mestranol 0.1 mg. were demonstrated in thirty-five women by direct inspection of the ovaries. When given alone, megestrol acetate 5 mg. or Mestranol 0.1 mg. did not prevent ovulation in all cases.
- ^ Schacter L, Rozencweig M, Canetta R, Kelley S, Nicaise C, Smaldone L (March 1989). "Megestrol acetate: clinical experience". Cancer Treat. Rev. 16 (1): 49–63. doi:10.1016/0305-7372(89)90004-2. PMID 2471590.
At 0.25 mg/day MA has no apparent effect on the histology of the endometrium and is not effective as a contraceptive (53). However, at doses of 0.35 and 0.5 mg/day the drug is an effective contraceptive (10). At the 0.5 mg/day dose MA does not inhibit ovulation but does reduce sperm motility in post-coital tests (68).
- ^ Vessey M, Mears E, Andolšek L, Ogrinc-Oven M (1972). "Randomised double-blind trial of four oral progestagen-only contraceptives". The Lancet. 299 (7757): 915–922. doi:10.1016/S0140-6736(72)91492-4. ISSN 0140-6736.
- ^ Aufrère MB, Benson H (June 1976). "Progesterone: an overview and recent advances". J Pharm Sci. 65 (6): 783–800. doi:10.1002/jps.2600650602. PMID 945344.
Early studies on its use as an oral contraceptive showed that, at 300 mg/day (5th to 25th day of the menstrual cycle), progesterone was effective in preventing ovulation through four cycles (263). The related effect of larger doses of progesterone on gonadotropin excretion also has been investigated. Rothchild (264) found that continuous or intermittent intravenously administered progesterone (100-400 mg/day) for 10 days depressed the total amount of gonadotropin excreted into the urine. However, Paulsen et al. (265) found that oral progesterone at 1000 mg/day for 87 days did not have a significant effect on urinary gonadotropin excretion. The efficacy of progesterone as an oral contraceptive was never fully tested, because synthetic progestational agents, which were orally effective, were available.
- ^ Pincus G (December 1958). "The hormonal control of ovulation and early development". Postgrad Med. 24 (6): 654–60. doi:10.1080/00325481.1958.11692305. PMID 13614060.
Table 1: Effects of oral progesterone on three indexes of ovulation: Medication: Progesterone. Number: 69. Mean cycle length: 25.5 ± 0.59. Per cent positive for ovulation by: Basal temperature: 27. Endometrial biopsy: 18. Vaginal smear: 6. [...] we settled on 300 mg. per day [oral progersterone] as a significantly effective [ovulation inhibition] dosage, and this was administered from the fifth day through the twenty-fourth day of the menstrual cycle. [...] We observed each of 33 volunteer subjects during a control, nontreatment cycle and for one to three successive cycles of medication immediately following the control cycle. As indexes of the occurrence of ovulation, daily basal temperatures and vaginal smears were taken, and at the nineteenth to twenty-second day of the cycle an endometrial biopsy. [...] Although we thus demonstrated the ovulation-inhibiting activity of progesterone in normally ovulating women, oral progesterone medication had two disadvantages: ( l) the large daily dosage ( 300 mg.) which presumably would have to be even larger if one sought 100 per cent inhibition1 [...]
- ^ Pincus G (1956). "Some effects of progesterone and related compounds upon reproduction and early development in mammals". Acta Endocrinol Suppl (Copenh). 23 (Suppl 28): 18–36. doi:10.1530/acta.0.023S018. PMID 13394044.
- ^ Stone A, Kupperman HS (1955). "The Effects of Progesterone on Ovulation: A Preliminary Report". The Fifth International Conference on Planned Parenthood: Theme, Overpopulation and Family Planning: Report of the Proceedings, 24-29 October, 1955, Tokyo, Japan. International Planned Parenthood Federation. p. 185.
- ^ S. Beier, B. Düsterberg, M. F. El Etreby, W. Elger, F. Neumann, Y. Nishino (1983). "Toxicology of Hormonal Fertility Regulating Agents". In Giuseppe Benagiano, Egon Diczfalusy (eds.). Endocrine Mechanisms in Fertility Regulation. Raven Press. pp. 261–346. ISBN 978-0-89004-464-3.
- ^ Knörr K, Beller FK, Lauritzen C (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. pp. 214–. ISBN 978-3-662-00942-0.
- ^ Knörr K, Knörr-Gärtner H, Beller FK, Lauritzen C (8 March 2013). Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion. Springer-Verlag. pp. 583–. ISBN 978-3-642-95583-9.
- ^ Labhart A (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 554–. ISBN 978-3-642-96158-8.
- ^ Horský J, Presl J (1981). "Hormonal Treatment of Disorders of the Menstrual Cycle". In Horsky J, Presl K (eds.). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 309–332. doi:10.1007/978-94-009-8195-9_11. ISBN 978-94-009-8195-9.
- ^ Ufer J (1969). The Principles and Practice of Hormone Therapy in Gynaecology and Obstetrics. de Gruyter. p. 49. ISBN 9783110006148.
17α-Hydroxyprogesterone caproate is a depot progestogen which is entirely free of side actions. The dose required to induce secretory changes in primed endometrium is about 250 mg. per menstrual cycle.
- ^ Pschyrembel W (1968). Praktische Gynäkologie: für Studierende und Ärzte. Walter de Gruyter. pp. 598, 601. ISBN 978-3-11-150424-7.
- ^ Ferin J (September 1972). "Effects, Duration of Action and Metabolism in Man". In Tausk M (ed.). Pharmacology of the Endocrine System and Related Drugs: Progesterone, Progestational Drugs and Antifertility Agents. Vol. II. Pergamon Press. pp. 13–24. ISBN 978-0080168128. OCLC 278011135.
- ^ Henzl MR, Edwards JA (10 November 1999). "Pharmacology of Progestins: 17α-Hydroxyprogesterone Derivatives and Progestins of the First and Second Generation". In Sitruk-Ware R, Mishell DR (eds.). Progestins and Antiprogestins in Clinical Practice. Taylor & Francis. pp. 101–132. ISBN 978-0-8247-8291-7.
- ^ Brotherton J (1976). Sex Hormone Pharmacology. Academic Press. p. 114. ISBN 978-0-12-137250-7.
- ^ Sang GW (April 1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–385. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.
- ^ Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- ^ Goebelsmann U (1986). "Pharmacokinetics of Contraceptive Steroids in Humans". In Gregoire AT, Blye RP (eds.). Contraceptive Steroids: Pharmacology and Safety. Springer Science & Business Media. pp. 67–111. doi:10.1007/978-1-4613-2241-2_4. ISBN 978-1-4613-2241-2.
- ^ Becker H, Düsterberg B, Klosterhalfen H (1980). "[Bioavailability of cyproterone acetate after oral and intramuscular application in men (author's transl)]" [Bioavailability of Cyproterone Acetate after Oral and Intramuscular Application in Men]. Urologia Internationalis. 35 (6): 381–385. doi:10.1159/000280353. PMID 6452729.
- ^ Moltz L, Haase F, Schwartz U, Hammerstein J (May 1983). "[Treatment of virilized women with intramuscular administration of cyproterone acetate]" [Efficacy of Intra muscularly Applied Cyproterone Acetate in Hyperandrogenism]. Geburtshilfe und Frauenheilkunde. 43 (5): 281–287. doi:10.1055/s-2008-1036893. PMID 6223851.
- ^ Wright JC, Burgess DJ (29 January 2012). Long Acting Injections and Implants. Springer Science & Business Media. pp. 114–. ISBN 978-1-4614-0554-2.
- ^ Chu YH, Li Q, Zhao ZF (April 1986). "Pharmacokinetics of megestrol acetate in women receiving IM injection of estradiol-megestrol long-acting injectable contraceptive". The Chinese Journal of Clinical Pharmacology.
The results showed that after injection the concentration of plasma MA increased rapidly. The meantime of peak plasma MA level was 3rd day, there was a linear relationship between log of plasma MA concentration and time (day) after administration in all subjects, elimination phase half-life t1/2β = 14.35 ± 9.1 days.
- ^ Runnebaum BC, Rabe T, Kiesel L (6 December 2012). Female Contraception: Update and Trends. Springer Science & Business Media. pp. 429–. ISBN 978-3-642-73790-9.
- ^ Artini PG, Genazzani AR, Petraglia F (11 December 2001). Advances in Gynecological Endocrinology. CRC Press. pp. 105–. ISBN 978-1-84214-071-0.
- ^ King TL, Brucker MC, Kriebs JM, Fahey JO (21 October 2013). Varney's Midwifery. Jones & Bartlett Publishers. pp. 495–. ISBN 978-1-284-02542-2.
- ^ de Lignières B, Silberstein S (April 2000). "Pharmacodynamics of oestrogens and progestogens". Cephalalgia: An International Journal of Headache. 20 (3): 200–7. doi:10.1046/j.1468-2982.2000.00042.x. PMID 10997774. S2CID 40392817.
- ^ Chassard D, Schatz B (2005). "[The antigonadrotropic activity of chlormadinone acetate in reproductive women]". Gynécologie, Obstétrique & Fertilité (in French). 33 (1–2): 29–34. doi:10.1016/j.gyobfe.2004.12.002. PMID 15752663.
- ^ a b Brady BM, Anderson RA, Kinniburgh D, Baird DT (April 2003). "Demonstration of progesterone receptor-mediated gonadotrophin suppression in the human male". Clinical Endocrinology. 58 (4): 506–12. doi:10.1046/j.1365-2265.2003.01751.x. PMID 12641635. S2CID 12567639.
- ^ Neumann F (1978). "The physiological action of progesterone and the pharmacological effects of progestogens--a short review". Postgraduate Medical Journal. 54 (Suppl 2): 11–24. PMID 368741.
- ^ Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA (25 August 2011). Campbell-Walsh Urology: Expert Consult Premium Edition: Enhanced Online Features and Print, 4-Volume Set. Elsevier Health Sciences. pp. 2938–. ISBN 978-1-4160-6911-9.
- ^ Kjeld JM, Puah CM, Kaufman B, Loizou S, Vlotides J, Gwee HM, Kahn F, Sood R, Joplin GF (1979). "Effects of norgestrel and ethinyloestradiol ingestion on serum levels of sex hormones and gonadotrophins in men". Clinical Endocrinology. 11 (5): 497–504. doi:10.1111/j.1365-2265.1979.tb03102.x. PMID 519881. S2CID 5836155.
- ^ Urotext (1 January 2001). Urotext-Luts: Urology. Urotext. pp. 71–. ISBN 978-1-903737-03-3.
- ^ Jacobi GH, Altwein JE, Kurth KH, Basting R, Hohenfellner R (1980). "Treatment of advanced prostatic cancer with parenteral cyproterone acetate: a phase III randomised trial". Br J Urol. 52 (3): 208–15. doi:10.1111/j.1464-410x.1980.tb02961.x. PMID 7000222.
- ^ a b c J. Horsky, J. Presl (6 December 2012). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 329–. ISBN 978-94-009-8195-9.
- ^ Bullock LP, Bardin CW (March 1977). "Androgenic, synandrogenic, and antiandrogenic actions of progestins". Annals of the New York Academy of Sciences. 286 (1 Biochemical A): 321–330. Bibcode:1977NYASA.286..321B. doi:10.1111/j.1749-6632.1977.tb29427.x. PMID 281183. S2CID 33611807.
- ^ a b c Darney PD (January 1995). "The androgenicity of progestins". The American Journal of Medicine. 98 (1A): 104S – 110S. doi:10.1016/S0002-9343(99)80067-9. PMID 7825629.
- ^ Campagnoli C, Clavel-Chapelon F, Kaaks R, Peris C, Berrino F (July 2005). "Progestins and progesterone in hormone replacement therapy and the risk of breast cancer". The Journal of Steroid Biochemistry and Molecular Biology. 96 (2): 95–108. doi:10.1016/j.jsbmb.2005.02.014. PMC 1974841. PMID 15908197.
- ^ Kenneth Hugdahl, René Westerhausen (2010). The Two Halves of the Brain: Information Processing in the Cerebral Hemispheres. MIT Press. pp. 272–. ISBN 978-0-262-01413-7.
- ^ a b Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH (December 2003). "Classification and pharmacology of progestins". Maturitas. 46 (Suppl 1): S7 – S16. doi:10.1016/j.maturitas.2003.09.014. PMID 14670641.
- ^ a b c David A. Williams, William O. Foye, Thomas L. Lemke (January 2002). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 700–. ISBN 978-0-683-30737-5.
- ^ a b c Ricardo Azziz (8 November 2007). Androgen Excess Disorders in Women. Springer Science & Business Media. pp. 124–. ISBN 978-1-59745-179-6.
- ^ a b P. J. Bentley (1980). Endocrine Pharmacology: Physiological Basis and Therapeutic Applications. CUP Archive. pp. 4–. ISBN 978-0-521-22673-8.
- ^ Sengupta (1 January 2007). Gynaecology For Postgraduate And Practitioners. Elsevier India. pp. 137–. ISBN 978-81-312-0436-8.
- ^ Ferin J (January 1962). "Artificial induction of hypo-oestrogenic amenorrhea with methylestrenolone, or with lynestrenol". Acta Endocrinologica. 39 (1): 47–67. doi:10.1530/acta.0.0390047. PMID 13892354.
- ^ Saunders FJ, Drill VA (May 1956). "The myotrophic and androgenic effects of 17-ethyl-19-nortestosterone and related compounds". Endocrinology. 58 (5): 567–572. doi:10.1210/endo-58-5-567. PMID 13317831.
- ^ a b c Armen H. Tashjian, Ehrin J. Armstrong (21 July 2011). Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Lippincott Williams & Wilkins. pp. 523–. ISBN 978-1-4511-1805-6.
- ^ de Gooyer ME, Deckers GH, Schoonen WG, Verheul HA, Kloosterboer HJ (January 2003). "Receptor profiling and endocrine interactions of tibolone". Steroids. 68 (1): 21–30. doi:10.1016/S0039-128X(02)00112-5. PMID 12475720. S2CID 40426061.
[Norethisterone] has similar and [norethynodrel] weaker androgenic effects compared to tibolone.
- ^ Raynaud JP, Ojasoo T (1986). "The design and use of sex-steroid antagonists". J. Steroid Biochem. 25 (5B): 811–33. doi:10.1016/0022-4731(86)90313-4. PMID 3543501.
Similar androgenic potential is inherent to norethisterone and its prodrugs (norethisterone acetate, ethynodiol diacetate, lynestrenol, norethynodrel, quingestanol).
- ^ a b Chaudhuri (1 January 2007). Practice Of Fertility Control: A Comprehensive Manual (7Th ed.). Elsevier India. pp. 122–. ISBN 978-81-312-1150-2.
- ^ Kuhl H (1996). "Comparative pharmacology of newer progestogens". Drugs. 51 (2): 188–215. doi:10.2165/00003495-199651020-00002. PMID 8808163. S2CID 1019532.
- ^ Offermanns S, Rosenthal W (14 August 2008). Encyclopedia of Molecular Pharmacology. Springer Science & Business Media. pp. 391–. ISBN 978-3-540-38916-3.
- ^ Lara Marks (2001). Sexual Chemistry: A History of the Contraceptive Pill. Yale University Press. pp. 73–75, 77–78. ISBN 978-0-300-08943-1.
- ^ Korn GW (March 1961). "The use of norethynodrel (enovid) in clinical practice". Canadian Medical Association Journal. 84 (11): 584–587. PMC 1939348. PMID 13753182.
Pseudohermaphroditism should not be a problem in these patients as it appears that norethynodrel does not possess androgenic properties, but it is believed that Wilkins has now found one such case in a patient who has been on norethynodrel therapy.
- ^ de Gooyer ME, Deckers GH, Schoonen WG, Verheul HA, Kloosterboer HJ (January 2003). "Receptor profiling and endocrine interactions of tibolone". Steroids. 68 (1): 21–30. doi:10.1016/s0039-128x(02)00112-5. PMID 12475720. S2CID 40426061.
- ^ de Ruggieri P, Matscher R, Lupo C, Spazzoli G (1965). "Biological properties of 17α-vinyl-5(10)-estrene-17β-ol-3-one (norvinodrel) as a progestational and claudogenic compound". Steroids. 5 (1): 73–91. doi:10.1016/0039-128X(65)90133-9. ISSN 0039-128X.
- ^ J. A. Simpson, E. S. C. Weiner (1997). Oxford English Dictionary Additions Series. Clarendon Press. pp. 36–. ISBN 978-0-19-860027-5.
- ^ JUCKER (8 March 2013). Fortschritte der Arzneimittelforschung / Progress in Drug Research / Progrès des recherches pharmaceutiques. Birkhäuser. pp. 166–. ISBN 978-3-0348-7053-5.
- ^ a b Annual Reports in Medicinal Chemistry. Academic Press. 8 September 1989. pp. 199–. ISBN 978-0-08-058368-6.
- ^ a b c Raudrant D, Rabe T (2003). "Progestogens with antiandrogenic properties". Drugs. 63 (5): 463–492. doi:10.2165/00003495-200363050-00003. PMID 12600226. S2CID 28436828.
- ^ Schneider HP (November 2003). "Androgens and antiandrogens". Annals of the New York Academy of Sciences. 997 (1): 292–306. Bibcode:2003NYASA.997..292S. doi:10.1196/annals.1290.033. PMID 14644837. S2CID 8400556.
- ^ Botella J, Paris J, Lahlou B (August 1987). "The cellular mechanism of the antiandrogenic action of nomegestrol acetate, a new 19-nor progestagen, on the rat prostate". Acta Endocrinologica. 115 (4): 544–550. doi:10.1530/acta.0.1150544. PMID 3630545.
- ^ Hammerstein J (1990). "Prodrugs: advantage or disadvantage?". Am. J. Obstet. Gynecol. 163 (6 Pt 2): 2198–203. doi:10.1016/0002-9378(90)90561-K. PMID 2256526.
- ^ a b Paulsen CA, Leach RB, Lanman J, Goldston N, Maddock WO, Heller CG (1962). "Inherent estrogenicity of norethindrone and norethynodrel: comparison with other synthetic progestins and progesterone". J. Clin. Endocrinol. Metab. 22 (10): 1033–9. doi:10.1210/jcem-22-10-1033. PMID 13942007.
- ^ a b Neumann F, Düsterberg B, Laurent H (1988). "Development of Progestogens". Female Contraception. pp. 129–140. doi:10.1007/978-3-642-73790-9_11. ISBN 978-3-642-73792-3.
- ^ a b Harvey PW (28 March 1996). Adrenal in Toxicology: Target Organ and Modulator of Toxicity. CRC Press. pp. 284–. ISBN 978-0-7484-0330-1.
- ^ Cuschieri A, Hanna G (20 January 2015). Essential Surgical Practice: Higher Surgical Training in General Surgery, Fifth Edition. CRC Press. pp. 899–. ISBN 978-1-4441-3763-7.
- ^ John A. Thomas (12 March 1997). Endocrine Toxicology, Second Edition. CRC Press. pp. 152–. ISBN 978-1-4398-1048-4.
- ^ Nick Panay, Paula Briggs, Gab Kovacs (20 August 2015). Managing the Menopause. Cambridge University Press. pp. 126–. ISBN 978-1-107-45182-7.
- ^ Meis PJ (May 2005). "17 hydroxyprogesterone for the prevention of preterm delivery". Obstetrics and Gynecology. 105 (5 Pt 1): 1128–1135. doi:10.1097/01.AOG.0000160432.95395.8f. PMID 15863556.
- ^ Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- ^ Louw-du Toit R, Hapgood JP, Africander D (May 2020). "A direct comparison of the transcriptional activities of progestins used in contraception and menopausal hormone therapy via the mineralocorticoid receptor". Biochem. Biophys. Res. Commun. 526 (2): 466–471. doi:10.1016/j.bbrc.2020.03.100. PMC 7287572. PMID 32234237.
- ^ Oelkers W (2002). "Antimineralocorticoid activity of a novel oral contraceptive containing drospirenone, a unique progestogen resembling natural progesterone". Eur J Contracept Reprod Health Care. 7 (Suppl 3): 19–26, discussion 42–3. PMID 12659403.
- ^ Foidart JM, Faustmann T (2007). "Advances in hormone replacement therapy: weight benefits of drospirenone, a 17alpha-spirolactone-derived progestogen". Gynecol. Endocrinol. 23 (12): 692–9. doi:10.1080/09513590701582323. PMID 18075844. S2CID 12572825.
- ^ Genazzani AR, Mannella P, Simoncini T (2007). "Drospirenone and its antialdosterone properties". Climacteric. 10 (Suppl 1): 11–8. doi:10.1080/13697130601114891. PMID 17364593. S2CID 24872884.
- ^ Palacios S, Foidart JM, Genazzani AR (2006). "Advances in hormone replacement therapy with drospirenone, a unique progestogen with aldosterone receptor antagonism". Maturitas. 55 (4): 297–307. doi:10.1016/j.maturitas.2006.07.009. hdl:2268/9932. PMID 16949774.
- ^ Blanton MP, Xie Y, Dangott LJ, Cohen JB (February 1999). "The steroid promegestone is a noncompetitive antagonist of the Torpedo nicotinic acetylcholine receptor that interacts with the lipid-protein interface". Mol. Pharmacol. 55 (2): 269–78. doi:10.1124/mol.55.2.269. PMID 9927618. S2CID 491327.
- ^ a b Neubauer H, Ma Q, Zhou J, Yu Q, Ruan X, Seeger H, Fehm T, Mueck AO (October 2013). "Possible role of PGRMC1 in breast cancer development". Climacteric. 16 (5): 509–13. doi:10.3109/13697137.2013.800038. PMID 23758160. S2CID 29808177.
- ^ Ruan X, Neubauer H, Yang Y, Schneck H, Schultz S, Fehm T, Cahill MA, Seeger H, Mueck AO (October 2012). "Progestogens and membrane-initiated effects on the proliferation of human breast cancer cells". Climacteric. 15 (5): 467–72. doi:10.3109/13697137.2011.648232. PMID 22335423. S2CID 11302554.
- ^ Trabert B, Sherman ME, Kannan N, Stanczyk FZ (September 2019). "Progesterone and breast cancer". Endocr. Rev. 41 (2): 320–344. doi:10.1210/endrev/bnz001. PMC 7156851. PMID 31512725.
- ^ a b c Fotherby K (August 1996). "Bioavailability of orally administered sex steroids used in oral contraception and hormone replacement therapy". Contraception. 54 (2): 59–69. doi:10.1016/0010-7824(96)00136-9. PMID 8842581.
- ^ Hargrove JT, Maxson WS, Wentz AC (October 1989). "Absorption of oral progesterone is influenced by vehicle and particle size". Am. J. Obstet. Gynecol. 161 (4): 948–51. doi:10.1016/0002-9378(89)90759-X. PMID 2801843.
- ^ Levine H, Watson N (March 2000). "Comparison of the pharmacokinetics of Crinone 8% administered vaginally versus Prometrium administered orally in postmenopausal women(3)". Fertil. Steril. 73 (3): 516–21. doi:10.1016/S0015-0282(99)00553-1. PMID 10689005.
- ^ Aufrère MB, Benson H (June 1976). "Progesterone: an overview and recent advances". J Pharm Sci. 65 (6): 783–800. doi:10.1002/jps.2600650602. PMID 945344.
- ^ a b Benno Clemens Runnebaum, Thomas Rabe, Ludwig Kiesel (6 December 2012). Female Contraception: Update and Trends. Springer Science & Business Media. pp. 429–. ISBN 978-3-642-73790-9.
- ^ a b Knörr K, Knörr-Gärtner H, Beller FK, Lauritzen C (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. pp. 214–. ISBN 978-3-662-00942-0.
- ^ a b Knörr K, Knörr-Gärtner H, Beller FK, Lauritzen C (8 March 2013). Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion. Springer-Verlag. pp. 583–. ISBN 978-3-642-95583-9.
- ^ a b A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 554–. ISBN 978-3-642-96158-8.
- ^ a b Horský J, Presl J (1981). "Hormonal Treatment of Disorders of the Menstrual Cycle". In Horsky J, Presl J (eds.). Ovarian Function and its Disorders. Springer Science & Business Media. pp. 309–332. doi:10.1007/978-94-009-8195-9_11. ISBN 978-94-009-8195-9.
- ^ Stanczyk FZ (2014). "Treatment of postmenopausal women with topical progesterone creams and gels: are they effective?". Climacteric. 17 (Suppl 2): 8–11. doi:10.3109/13697137.2014.944496. PMID 25196424. S2CID 20019151.
- ^ Stanczyk FZ, Paulson RJ, Roy S (2005). "Percutaneous administration of progesterone: blood levels and endometrial protection". Menopause. 12 (2): 232–7. doi:10.1097/00042192-200512020-00019. PMID 15772572. S2CID 10982395.
- ^ Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH (2008). "Classification and pharmacology of progestins" (PDF). Maturitas. 61 (1–2): 171–80. doi:10.1016/j.maturitas.2008.11.013. PMID 19434889. [permanent dead link]
- ^ Edgren RA, Stanczyk FZ (December 1999). "Nomenclature of the gonane progestins". Contraception. 60 (6): 313. doi:10.1016/s0010-7824(99)00101-8. PMID 10715364.
- ^ Inhoffen HH, Logemann W, Hohlweg W, Serini A (May 4, 1938). "Untersuchungen in der Sexualhormon-Reihe (Investigations in the sex hormone series)". Ber Dtsch Chem Ges. 71 (5): 1024–32. doi:10.1002/cber.19380710520. Archived from the original on December 17, 2012.
- ^ a b c Maisel, Albert Q. (1965). The Hormone Quest. New York: Random House. OCLC 543168.
- ^ a b c Petrow V (1970). "The contraceptive progestagens". Chem Rev. 70 (6): 713–26. doi:10.1021/cr60268a004. PMID 4098492.
- ^ a b c Sneader, Walter (2005). "Hormone analogues". Drug discovery: a history. Hoboken, NJ: John Wiley & Sons. pp. 188–225. ISBN 978-0-471-89980-8.
- ^ a b c Djerassi C (2006). "Chemical birth of the pill". Am J Obstet Gynecol. 194 (1): 290–8. doi:10.1016/j.ajog.2005.06.010. PMID 16389046.
- ^ Djerassi C, Miramontes L, Rosenkranz G, Sondheimer F (1954). "Steroids. LIV. Synthesis of 19-Nor-17α-ethynyltestosterone and 19-Nor-17α-methyltestosterone". J Am Chem Soc. 76 (16): 4089–91. doi:10.1021/ja01645a009.
- ^ Colton FB (1992). "Steroids and "the pill": early steroid research at Searle". Steroids. 57 (12): 624–30. doi:10.1016/0039-128X(92)90015-2. PMID 1481226. S2CID 28718601.
- ^ Creinin MD, Jensen JT (September 2020). "Oral contraceptive generations - Time to stop using a marketing myth to define nomenclature". Contraception. 102 (3): 143–144. doi:10.1016/j.contraception.2020.05.017. PMID 32504633. S2CID 219529452.
- ^ a b c Nieschlag E (2010). "Clinical trials in male hormonal contraception" (PDF). Contraception. 82 (5): 457–70. doi:10.1016/j.contraception.2010.03.020. PMID 20933120.
- ^ C. Coutifaris, L. Mastroianni (15 August 1997). New Horizons in Reproductive Medicine. CRC Press. pp. 101–. ISBN 978-1-85070-793-6.
- ^ Shio Kumar Singh (4 September 2015). Mammalian Endocrinology and Male Reproductive Biology. CRC Press. pp. 270–. ISBN 978-1-4987-2736-5.
- ^ Frick J (1973). "Control of spermatogenesis in men by combined administration of progestin and androgen". Contraception. 8 (3): 191–206. doi:10.1016/0010-7824(73)90030-9. ISSN 0010-7824.
- ^ Nieschlag E, Kumar N, Sitruk-Ware R (2013). "7α-methyl-19-nortestosterone (MENTR): the population council's contribution to research on male contraception and treatment of hypogonadism". Contraception. 87 (3): 288–95. doi:10.1016/j.contraception.2012.08.036. PMID 23063338.
- ^ Attardi BJ, Hild SA, Reel JR (2006). "Dimethandrolone undecanoate: a new potent orally active androgen with progestational activity". Endocrinology. 147 (6): 3016–26. doi:10.1210/en.2005-1524. PMID 16497801.
- ^ Foegh M (1983). "Evaluation of steroids as contraceptives in men". Acta Endocrinol Suppl (Copenh). 260 (3_Supplb): 3–48. doi:10.1530/acta.0.104S009. PMID 6415998.
At the time our studies were initiated, 11 different gestagens have been tested in men. All the oral preparations were used in doses 5 to 12 fold that used in the female oral contraceptive. The only exception was levo-norgestrel which was used in a very low dose, namely 100 µg daily (Fotherby et al. 1972). However, no effect was obtained on sperm count and in vitro sperm penetration.
Further reading
- Kuhl H (September 1990). "Pharmacokinetics of oestrogens and progestogens". Maturitas. 12 (3): 171–97. doi:10.1016/0378-5122(90)90003-o. PMID 2170822.
- Lauritzen C (September 1990). "Clinical use of oestrogens and progestogens". Maturitas. 12 (3): 199–214. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- Stanczyk FZ (September 2002). "Pharmacokinetics and potency of progestins used for hormone replacement therapy and contraception". Rev Endocr Metab Disord. 3 (3): 211–24. doi:10.1023/A:1020072325818. PMID 12215716. S2CID 27018468.
- Raudrant D, Rabe T (2003). "Progestogens with antiandrogenic properties". Drugs. 63 (5): 463–92. doi:10.2165/00003495-200363050-00003. PMID 12600226. S2CID 28436828.
- Stanczyk FZ (November 2003). "All progestins are not created equal". Steroids. 68 (10–13): 879–90. doi:10.1016/j.steroids.2003.08.003. PMID 14667980. S2CID 44601264.
- Nieschlag E, Zitzmann M, Kamischke A (November 2003). "Use of progestins in male contraception". Steroids. 68 (10–13): 965–72. doi:10.1016/S0039-128X(03)00135-1. PMID 14667989. S2CID 22458746.
- Wiegratz I, Kuhl H (August 2004). "Progestogen therapies: differences in clinical effects?". Trends Endocrinol. Metab. 15 (6): 277–85. doi:10.1016/j.tem.2004.06.006. PMID 15358281. S2CID 35891204.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- Sitruk-Ware R (October 2005). "Pharmacology of different progestogens: the special case of drospirenone". Climacteric. 8 (Suppl 3): 4–12. doi:10.1080/13697130500330382. PMID 16203650. S2CID 24205704.
- Wiegratz I, Kuhl H (September 2006). "Metabolic and clinical effects of progestogens". Eur J Contracept Reprod Health Care. 11 (3): 153–61. doi:10.1080/13625180600772741. PMID 17056444. S2CID 27088428.
- Stanczyk FZ (2007). "Structure –Function Relationships, Pharmacokinetics, and Potency of Orally and Parenterally Administered Progestogens". Treatment of the Postmenopausal Woman. pp. 779–798. doi:10.1016/B978-012369443-0/50067-3. ISBN 978-0-12-369443-0.
- Sitruk-Ware R, Nath A (November 2010). "The use of newer progestins for contraception". Contraception. 82 (5): 410–7. doi:10.1016/j.contraception.2010.04.004. PMID 20933114.
- Kuhl H (2011). "Pharmacology of progestogens" (PDF). Journal für Reproduktionsmedizin und Endokrinologie-Journal of Reproductive Medicine and Endocrinology. 8 (Special Issue 1): 157–176.
- Lawrie TA, Helmerhorst FM, Maitra NK, Kulier R, Bloemenkamp K, Gülmezoglu AM (May 2011). "Types of progestogens in combined oral contraception: effectiveness and side-effects". Cochrane Database Syst Rev (5): CD004861. doi:10.1002/14651858.CD004861.pub2. PMID 21563141.
- Endrikat J, Gerlinger C, Richard S, Rosenbaum P, Düsterberg B (December 2011). "Ovulation inhibition doses of progestins: a systematic review of the available literature and of marketed preparations worldwide". Contraception. 84 (6): 549–57. doi:10.1016/j.contraception.2011.04.009. PMID 22078182.
- Moore NL, Hickey TE, Butler LM, Tilley WD (June 2012). "Multiple nuclear receptor signaling pathways mediate the actions of synthetic progestins in target cells". Mol. Cell. Endocrinol. 357 (1–2): 60–70. doi:10.1016/j.mce.2011.09.019. PMID 21945474. S2CID 20006148.
- Stanczyk FZ, Hapgood JP, Winer S, Mishell DR (April 2013). "Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects". Endocr. Rev. 34 (2): 171–208. doi:10.1210/er.2012-1008. PMC 3610676. PMID 23238854.
- Grimes DA, Lopez LM, O'Brien PA, Raymond EG (November 2013). "Progestin-only pills for contraception". Cochrane Database Syst Rev (11): CD007541. doi:10.1002/14651858.CD007541.pub3. PMID 24226383.
- Hapgood JP, Africander D, Louw R, Ray RM, Rohwer JM (July 2014). "Potency of progestogens used in hormonal therapy: toward understanding differential actions". J. Steroid Biochem. Mol. Biol. 142: 39–47. doi:10.1016/j.jsbmb.2013.08.001. PMID 23954501. S2CID 12142015.
- Sitruk-Ware R, El-Etr M (August 2013). "Progesterone and related progestins: potential new health benefits". Climacteric. 16 (Suppl 1): 69–78. doi:10.3109/13697137.2013.802556. PMID 23647429. S2CID 25447915.
- Bińkowska M, Woroń J (June 2015). "Progestogens in menopausal hormone therapy". Prz Menopauzalny. 14 (2): 134–43. doi:10.5114/pm.2015.52154. PMC 4498031. PMID 26327902.