Pixantrone
| |
| Names | |
|---|---|
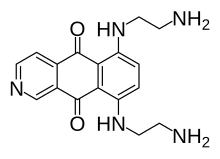
| Preferred IUPAC name 6,9-Bis[(2-aminoethyl)amino]benzo[g]isoquinoline-5,10-dione | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C17H19N5O2 | |
| Molar mass | 325.365 g/mol |
| Appearance | Blue solid |
| Pharmacology | |
| L01DB11 (WHO) | |
| Intravenous | |
| Pharmacokinetics: | |
| 9.5–17.5 hours | |
| Fecal (main route of excretion) and renal (4–9%) | |
| Legal status | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Pixantrone (rINN; trade name Pixuvri) is an experimental antineoplastic (anti-cancer) drug, an analogue of mitoxantrone with fewer toxic effects on cardiac tissue.[3] It acts as a topoisomerase II poison and intercalating agent.[4][5] The code name BBR 2778 refers to pixantrone dimaleate, the actual substance commonly used in clinical trials.[6]
History
Anthracyclines are important chemotherapy agents. However, their use is associated with irreversible and cumulative heart damage. Investigators have attempted to design related drugs that maintain the biological activity, but do not possess the cardiotoxicity of the anthracyclines.[7] Pixantrone was developed to reduce heart damage related to treatment while retaining efficacy.[3]
Random screening at the US National Cancer Institute of a vast number of compounds provided by the Allied Chemical Company led to the discovery of ametantrone as having significant anti-tumor activity. Further investigation regarding the rational development of analogs of ametantrone led to the synthesis of mitoxantrone, which also exhibited marked anti-tumor activity[7] Mitoxantrone was considered as an analog of doxorubicin with less structural complexity but with a similar mode of action. In clinical studies, mitoxantrone was shown to be effective against numerous types of tumors with less toxic side effects than those resulting from doxorubicin therapy. However, mitoxantrone was not totally free of cardiotoxicity. A number of structurally modified analogs of mitoxantrone were synthesized and structure-activity relationship studies made.[7] BBR 2778 was originally synthesized by University of Vermont researchers Miles P. Hacker and Paul A. Krapcho[7] and initially characterized in vitro for tumor cell cytotoxicity and mechanism of action by studies at the Boehringer Mannheim Italia Research Center, Monza, and University of Vermont, Burlington.[6] Other studies have been completed at the University of Texas M. D. Anderson Cancer Center, Houston, the Istituto Nazionale Tumori, Milan, and the University of Padua.[4][6][8] In the search for novel heteroanalogs of anthracenediones, it was selected as the most promising compound. Toxicological studies indicated that BBR 2778 was not cardiotoxic, and US patents are held by the University of Vermont. An additional US patent application was completed in June 1995 by Boehringer Mannheim, Italy.[7]
Novuspharma, an Italian company, was established in 1998 following the merger of Boehringer Mannheim and Hoffmann-La Roche, and BBR 2778 was developed as Novuspharma's leading anti-cancer drug, pixantrone.[9] A patent application for the injectable preparation was filed in May 2003.[10]
In 2003, Cell Therapeutics, a Seattle biotechnology company, acquired pixantrone through a merger with Novuspharma.[11]
Clinical trials
Pixantrone is a substance that is being studied in the treatment of cancer. It belongs to the family of drugs called antitumor antibiotics.[12] phase III clinical trials of pixantrone have been completed.[13][14] Pixantrone is being studied as an antineoplastic for different kinds of cancer, including solid tumors and hematological malignancies such as non-Hodgkin lymphomas.
Animal studies demonstrated that pixantrone does not worsen pre-existing heart muscle damage, suggesting that pixantrone may be useful in patients pretreated with anthracyclines. While only minimal cardiac changes are observed in mice given repeated cycles of pixantrone, 2 cycles of traditional anthracyclines doxorubicin or mitoxantrone result in marked or severe heart muscle degeneration.[3]
Clinical trials substituting pixantrone for doxorubicin in standard first-line treatment of patients with aggressive non-Hodgkin's lymphoma, had a reduction in severe side effects when compared to patients treated with standard doxorubicin-based therapy. Despite pixantrone patients receiving more treatment cycles, a three-fold reduction in the incidence of severe heart damage was seen as well as clinically significant reductions in infections and thrombocytopenia, and a significant reduction in febrile neutropenia. These findings could have major implications for treating patients with breast cancer, lymphoma, and leukemia, where debilitating cardiac damage from doxorubicin might be prevented.[15] Previous treatment options for multiply relapsed aggressive non-Hodgkin lymphoma had disappointing response rates.[16]
The completed phase II RAPID trial compared the CHOP-R regimen of Cyclophosphamide, Doxorubicin, Vincristine, Prednisone, and Rituximab to the same regimen, but substituting Doxorubicin with Pixantrone. The objective was to show that Pixantrone was not inferior to Doxorubicin and less toxic to the heart.[17]
Pixantrone was shown to have potentially reduced cardiotoxicity and demonstrated promising clinical activity in these phase II studies in heavily pretreated non-Hodgkin lymphoma patients.[16]
The phase III EXTEND (PIX301) randomized clinical trial studied pixantrone to see how well it works compared to other chemotherapy drugs in treating participants with relapsed non-Hodgkin's lymphoma.[18] The complete response rate in pixantrone treated with pixantrone has been significantly higher than in those receiving other chemotherapeutic agents for treatment of relapsed/refractory aggressive non-Hodgkin lymphoma.[16]
Administration
It can be administered through a peripheral vein rather than a central implanted catheter as required for other similar drugs.[10][16]
Society and culture
Legal status
US Food and Drug Administration
The US Food and Drug Administration (FDA) granted fast track designation for pixantrone in people who had previously been treated two or more times for relapsed or refractory aggressive Non-Hodgkin's Lymphoma. Study sponsor Cell Therapeutics announced that pixantrone achieved the primary efficacy endpoint. The minutes of the Oncologic Drugs Advisory Committee meeting of 22 March 2010[19] show that this had not in fact been achieved with statistical significance and this combined with major safety concerns lead to the conclusion that the trial was not sufficient to support approval. In April 2010, the FDA asked for an additional trial.[20]
European Medicines Agency
In May 2009, pixantrone became available in the European Union on a named-patient basis. A named-patient program is a compassionate use drug supply program under which physicians can legally supply investigational drugs to qualifying individuals. Under a named-patient program, investigational drugs can be administered to people who are suffering from serious illnesses prior to the drug being approved by the European Medicines Evaluation Agency. "Named-patient" distribution refers to the distribution or sale of a product to a specific healthcare professional for the treatment of an individual. In the European Union, under the named-patient program the drug is most often purchased through the national health system.[21] In 2012, pixantrone received conditional marketing authorization in the European Union as Monotherapy to Treat Adult Patients with Multiply Relapsed or Refractory Aggressive Non-Hodgkin B-Cell Lymphomas.[22][2] The authorization expired in July 2024, after the holder decided not to renew.[2]
Research
Pixantrone is as potent as mitoxantrone in animal models of multiple sclerosis.[23] Pixantrone has a similar mechanism of action as mitoxantrone on the effector function of lymphomonocyte B and T cells in experimental allergic encephalomyelitis but with lower cardiotoxicity. Pixantrone inhibits antigen specific and mitogen induced lymphomononuclear cell proliferation, as well as IFN-gamma production.[24] Clinical trials are currently ongoing in Europe.
Pixantrone also reduces the severity of experimental autoimmune myasthenia gravis in Lewis rats,[25] and in vitro cell viability experiments indicated that Pixantrone significantly reduces amyloid beta (A beta(1-42)) neurotoxicity, a mechanism implicated in Alzheimer's disease.[26]
References
- ^ "Pixuvri 29 mg powder for concentrate for solution for infusion". (emc). 23 November 2021. Retrieved 12 July 2024.
- ^ a b c "Pixuvri EPAR". European Medicines Agency. 31 May 2012. Retrieved 12 July 2024.
- ^ a b c Cavalletti E, Crippa L, Mainardi P, Oggioni N, Cavagnoli R, Bellini O, et al. (2007). "Pixantrone (BBR 2778) has reduced cardiotoxic potential in mice pretreated with doxorubicin: comparative studies against doxorubicin and mitoxantrone". Invest New Drugs. 25 (3): 187–95. doi:10.1007/s10637-007-9037-8. PMID 17285358. S2CID 20933712.
- ^ a b De Isabella P, Palumbo M, Sissi C, Capranico G, Carenini N, Menta E, et al. (1995). "Topoisomerase II DNA cleavage stimulation, DNA binding activity, cytotoxicity, and physico-chemical properties of 2-aza- and 2-aza-oxide-anthracenedione derivatives". Mol. Pharmacol. 48 (1): 30–8. PMID 7623772.
- ^ Evison BJ, Mansour OC, Menta E, Phillips DR, Cutts SM (2007). "Pixantrone can be activated by formaldehyde to generate a potent DNA adduct forming agent". Nucleic Acids Res. 35 (11): 3581–9. doi:10.1093/nar/gkm285. PMC 1920253. PMID 17483512.
- ^ a b c Krapcho AP, Petry ME, Getahun Z, Landi JJ Jr, Stallman J, Polsenberg JF, et al. (1994). "6,9-Bis[(aminoalkyl)amino]benzo[g]isoquinoline-5,10-diones. A novel class of chromophore-modified antitumor anthracene-9,10-diones: synthesis and antitumor evaluations". J Med Chem. 37 (6): 828–37. doi:10.1021/jm00032a018. PMID 8145234.
- ^ a b c d e US patent 5587382, Krapcho AP, Hacker MP, Cavalletti E, Giuliani FC, "6,9-bis[(2-aminoethyl) amino]benzo [g]isoquinoline-5,10- dione dimaleate; an aza-anthracenedione with reduced cardiotoxicity", issued 24 December 1996, assigned to Boehringer Mannheim Italia, SpA
- ^ Zwelling LA, Mayes J, Altschuler E, Satitpunwaycha P, Tritton TR, Hacker MP (1993). "Activity of two novel anthracene-9,10-diones against human leukemia cells containing intercalator-sensitive or -resistant forms of topoisomerase II". Biochem. Pharmacol. 46 (2): 265–71. doi:10.1016/0006-2952(93)90413-Q. PMID 8394077.
- ^ Borchmann P, Reiser M (May 2003). "Pixantrone (Novuspharma)". IDrugs. 6 (5): 486–90. PMID 12789604.
- ^ a b EP patent 1503797, Bernareggi A, Livi V, "Injectable Pharmaceutical Compositions of an Anthracenedione Derivative with Anti-Tumoral Activity", published 27 November 2003, issued 2008-09-29, assigned to Cell Therapeutics Europe S.R.L.
- ^ Pollack A (17 June 2003). "Company News; Cell Therapeutics Announces Plan To Buy Novuspharma". The New York Times. Retrieved 22 May 2010.
- ^ Mosby's Medical Dictionary, 8th edition. © 2009, Elsevier. "definition of antineoplastic antibiotic". Free Online Medical Dictionary, Thesaurus and Encyclopedia. Retrieved 31 January 2012.
{{cite web}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ "NCT00088530". BBR 2778 for Relapsed, Aggressive Non-Hodgkin's Lymphoma (NHL). ClinicalTrials.gov. Retrieved 31 January 2012.
- ^ "NCT00551239". Fludarabine and Rituximab With or Without Pixantrone in Treating Patients With Relapsed or Refractory Indolent Non-Hodgkin Lymphoma. ClinicalTrials.gov. 31 January 2012. Retrieved 31 January 2012.
- ^ "Pixantrone Combination Therapy for First-line Treatment of Aggressive Non-Hodgkin's Lymphoma Results in Reduction in Severe Toxicities Including Heart Damage When Compared to Doxorubicin-based Therapy". Press Release (Press release). Retrieved 31 January 2012.
- ^ a b c d Engert A, Herbrecht R, Santoro A, Zinzani PL, Gorbatchevsky I (September 2006). "EXTEND PIX301: a phase III randomized trial of pixantrone versus other chemotherapeutic agents as third-line monotherapy in patients with relapsed, aggressive non-Hodgkin's lymphoma". Clin Lymphoma Myeloma. 7 (2): 152–4. doi:10.3816/CLM.2006.n.055. PMID 17026830.
- ^ "NCT00268853". A Trial in Patients With Diffuse Large-B-cell Lymphoma Comparing Pixantrone Against Doxorubicin. ClinicalTrials.gov. Retrieved 31 January 2012.
- ^ "NCT00101049". BBR 2778 for Relapsed, Aggressive Non-Hodgkin's Lymphoma (NHL). ClinicalTrials.gov. Retrieved 31 January 2012.
- ^ Vesely N, Eckhardt SG (22 March 2010). "NDA 022-481 PIXUVRI (pixantrone dimaleate) injection" (PDF). Summary Minutes of the Oncologic Drugs Advisory Committee. United States Food and Drug Administration. Retrieved 31 January 2012.
- ^ "Cell Therapeutics Formally Appeals FDA's Nonapprovable Ruling for Pixantrone". GEN News. 3 December 2010.
- ^ "Pixantrone Now Available in Europe on a Named-Patient Basis". Archived from the original on 1 October 2009. Retrieved 31 January 2012.
- ^ Péan E, Flores B, Hudson I, Sjöberg J, Dunder K, Salmonson T, et al. (2013). "The European Medicines Agency review of pixantrone for the treatment of adult patients with multiply relapsed or refractory aggressive non-Hodgkin's B-cell lymphomas: summary of the scientific assessment of the committee for medicinal products for human use". The Oncologist. 18 (5): 625–33. doi:10.1634/theoncologist.2013-0020. PMC 3662855. PMID 23615696.
- ^ Gonsette RE, Dubois B (August 2004). "Pixantrone (BBR2778): a new immunosuppressant in multiple sclerosis with a low cardiotoxicity". J. Neurol. Sci. 223 (1): 81–6. doi:10.1016/j.jns.2004.04.024. PMID 15261566. S2CID 35170743.
- ^ Mazzanti B, Biagioli T, Aldinucci A, Cavaletti G, Cavalletti E, Oggioni N, et al. (November 2005). "Effects of pixantrone on immune-cell function in the course of acute rat experimental allergic encephalomyelitis". J. Neuroimmunol. 168 (1–2): 111–7. doi:10.1016/j.jneuroim.2005.07.010. PMID 16120465. S2CID 32913479.
- ^ Ubiali F, Nava S, Nessi V, Longhi R, Pezzoni G, Capobianco R, et al. (February 2008). "Pixantrone (BBR2778) reduces the severity of experimental autoimmune myasthenia gravis in Lewis rats". J. Immunol. 180 (4): 2696–703. doi:10.4049/jimmunol.180.4.2696. PMID 18250482.
- ^ Colombo R, Carotti A, Catto M, Racchi M, Lanni C, Verga L, et al. (April 2009). "CE can identify small molecules that selectively target soluble oligomers of amyloid beta protein and display antifibrillogenic activity". Electrophoresis. 30 (8): 1418–29. doi:10.1002/elps.200800377. PMID 19306269. S2CID 6594113.
å
