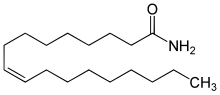
Oleamide
| |
| Names | |
|---|---|
| Preferred IUPAC name (9Z)-Octadec-9-enamide | |
| Other names oleoyl-amide Oleylamide 9-Octadecenamide (Z)-9-Octadecenamide 9,10-Octadecenoamide Oleic acid amide Cis-9,10-octadecenoamide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.550 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H35NO | |
| Molar mass | 281.484 g·mol−1 |
| Appearance | Creamy solid[1] |
| Density | 0.879 g/cm3 |
| Melting point | 70 °C (158 °F; 343 K)<[2] |
| Boiling point | > 200 °C (392 °F; 473 K)[1] |
| Insoluble[1] | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | > 200 °C (392 °F; 473 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Oleamide is an organic compound with the formula CH3(CH2)7CH=CH(CH2)7CONH2.[3] It is the amide derived from the fatty acid oleic acid. It is a colorless waxy solid and occurs in nature. Sometimes labeled as a fatty acid primary amide (FAPA), it is biosynthesized from N-oleoylglycine.[4]
Biochemical and medical aspects
In terms of natural occurrence, oleamide was first detected in human plasma. It was later shown to accumulate in the cerebrospinal fluid during sleep deprivation and induces sleep in animals.[4][5]
It has been considered as a treatment for mood and sleep disorders, as well as cannabinoid-regulated depression.[6][7]
In terms of its sleep inducing effects, it is speculated that oleamide interacts with multiple neurotransmitter systems.[8][9] Some in-vitro studies show that cis-oleamide is an agonist for the cannabinoid receptor CB-1 with an affinity around 8 micromolar.[10] However, given oleamide's relatively low affinity for CB-1 and uncertainty about the concentration and biological role of oleamide in-vivo, it has been argued that it is premature to classify oleamide as an endocannabinoid.[11] At larger doses oleamide can lower the body temperature of mice by about 2 degrees, with the effect lasting about two hours.[9] The mechanism for this remains unknown.[9]
Oleamide has been found to enhance PPARα-dependent increase in doublecortin, a marker of neurogenesis in the hippocampus[12]
Oleamide is rapidly metabolized by fatty acid amide hydrolase (FAAH), the same enzyme that metabolizes anandamide.[13] It has been postulated that some effects of oleamide are caused by increased concentrations of anandamide brought about through the inhibition of FAAH.[9]
It has been claimed that oleamide increases the activity of choline acetyltransferase, an enzyme that is critical in the production of acetylcholine.[14]
Other occurrences
Oleamide has been found in Ziziphus jujuba, also known as Jujube fruit.[14]
Synthetic oleamide has a variety of industrial uses, including as a lubricant.[15]
Oleamide was found to be leaching out of polypropylene plastics in laboratory experiments, affecting experimental results.[16] Since polypropylene is used in a wide number of food containers such as those for yogurt, the problem is being studied.[17]
Oleamide is "one of the most frequent non-cannabinoid ingredients associated with Spice products."[18] Analysis of 44 products synthetic cannabinoid revealed oleamide in 7 of the products tested.[19]
See also
References
- ^ a b c d Oleamide at chemicalland21.com
- ^ "(9Z)-9-Octadecenamide | C18H35NO | ChemSpider".
- ^ "Oleamide".
- ^ a b McKinney, Michele K.; Cravatt, Benjamin F. (June 2005). "Structure and Function of Fatty Acid Amide Hydrolase". Annual Review of Biochemistry. 74 (1): 411–432. doi:10.1146/annurev.biochem.74.082803.133450. PMID 15952893. S2CID 23571858.
- ^ Cravatt, B.; Prospero-Garcia, O; Siuzdak, G; Gilula, N.; Henriksen, S.; Boger, D.; Lerner, R. (9 June 1995). "Chemical characterization of a family of brain lipids that induce sleep". Science. 268 (5216): 1506–1509. Bibcode:1995Sci...268.1506C. doi:10.1126/science.7770779. PMID 7770779. S2CID 32070839.
- ^ Methods of treating anxiety and mood disorders with oleamide – US Patent 6359010 Archived 2011-06-12 at the Wayback Machine
- ^ Mechoulam, Raphael; Fride, Ester; Hanu, Lumir; Sheskin, Tzviel; Bisogno, Tiziana; Di Marzo, Vincenzo; Bayewitch, Michael; Vogel, Zvi (September 1997). "Anandamide may mediate sleep induction". Nature. 389 (6646): 25–26. Bibcode:1997Natur.389R..25M. doi:10.1038/37891. PMID 9288961. S2CID 26371103.
- ^ Fedorova, I; Hashimoto, A; Fecik, RA; Hedrick, MP; Hanus, LO; Boger, DL; Rice, KC; Basile, AS (October 2001). "Behavioral evidence for the interaction of oleamide with multiple neurotransmitter systems". The Journal of Pharmacology and Experimental Therapeutics. 299 (1): 332–42. PMID 11561096.
- ^ a b c d Hiley, C. Robin; Hoi, Pui Man (13 April 2007). "Oleamide: A Fatty Acid Amide Signaling Molecule in the Cardiovascular System?: OLEAMIDE". Cardiovascular Drug Reviews. 25 (1): 46–60. doi:10.1111/j.1527-3466.2007.00004.x. PMID 17445087.
- ^ Leggett, JD; Aspley, S; Beckett, SR; D'Antona, AM; Kendall, DA; Kendall, DA (January 2004). "Oleamide is a selective endogenous agonist of rat and human CB1 cannabinoid receptors". British Journal of Pharmacology. 141 (2): 253–62. doi:10.1038/sj.bjp.0705607. PMC 1574194. PMID 14707029.
- ^ Fowler, Christopher J (January 2004). "Oleamide: a member of the endocannabinoid family?: Commentary". British Journal of Pharmacology. 141 (2): 195–196. doi:10.1038/sj.bjp.0705608. PMC 1574195. PMID 14691053.
- ^ Roy, Avik; Kundu, Madhuchhanda; Chakrabarti, Sudipta; Patel, Dhruv R; Pahan, Kalipada (December 2021). "Oleamide, a Sleep-Inducing Supplement, Upregulates Doublecortin in Hippocampal Progenitor Cells via PPARα". J Alzheimers Dis. 84 (4): 1747–1762. doi:10.3233/JAD-215124. PMC 10075226. PMID 34744082.
- ^ Maurelli, Stefano; Bisogno, Tiziana; De Petrocellis, Luciano; Di Luccia, Aldo; Marino, Gennaro; Di Marzo, Vincenzo (11 December 1995). "Two novel classes of neuroactive fatty acid amides are substrates for mouse neuroblastoma 'anandamide amidohydrolase'". FEBS Letters. 377 (1): 82–86. Bibcode:1995FEBSL.377...82M. doi:10.1016/0014-5793(95)01311-3. PMID 8543025. S2CID 7461775.
- ^ a b Heo, Ho-Jin; Park, Young-June; Suh, Young-Min; Choi, Soo-Jung; Kim, Mi-Jeong; Cho, Hong-Yon; Chang, Yun-Jeong; Hong, Bumshik; Kim, Hye-Kyung; Kim, Eunki; Kim, Chang-Ju; Kim, Byung-Gee; Shin, Dong-Hoon (January 2003). "Effects of Oleamide on Choline Acetyltransferase and Cognitive Activities". Bioscience, Biotechnology, and Biochemistry. 67 (6): 1284–1291. doi:10.1271/bbb.67.1284. PMID 12843655.
- ^ Surfactants : Westco Oleamide a Slip Agent In Polyethylene Films Archived January 27, 2007, at the Wayback Machine
- ^ McDonald, G. R.; Hudson, A. L.; Dunn, S. M. J.; You, H.; Baker, G. B.; Whittal, R. M.; Martin, J. W.; Jha, A.; Edmondson, D. E.; Holt, A. (7 November 2008). "Bioactive Contaminants Leach from Disposable Laboratory Plasticware". Science. 322 (5903): 917. Bibcode:2008Sci...322..917M. doi:10.1126/science.1162395. PMID 18988846. S2CID 35526901.
- ^ Mittelstaedt, Martin (6 November 2008). "Researchers Raise Alarm After Chemical Leak Found In Common Plastic". Globe and Mail. Retrieved 10 June 2013.
- ^ Fattore, Liana; Fratta, Walter (21 September 2011). "Beyond THC: The New Generation of Cannabinoid Designer Drugs". Frontiers in Behavioral Neuroscience. 5: 60. doi:10.3389/fnbeh.2011.00060. PMC 3187647. PMID 22007163.
- ^ Uchiyama, Nahoko; Kikura-Hanajiri, Ruri; Ogata, Jun; Goda, Yukihiro (May 2010). "Chemical analysis of synthetic cannabinoids as designer drugs in herbal products". Forensic Science International. 198 (1–3): 31–38. doi:10.1016/j.forsciint.2010.01.004. PMID 20117892.

