Odevixibat
 | |
| Clinical data | |
|---|---|
| Trade names | Bylvay |
| Other names | A4250 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a621049 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
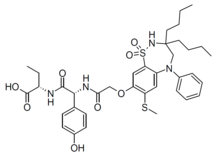
| Formula | C37H48N4O8S2 |
| Molar mass | 740.93 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Odevixibat, sold under the brand name Bylvay among others, is a medication for the treatment of progressive familial intrahepatic cholestasis.[5][9] It is taken by mouth.[5] Odevixibat is a reversible, potent, selective inhibitor of the ileal bile acid transporter (IBAT).[9][10][11] It was developed by Albireo Pharma.[12]
The most common side effects include diarrhea, abdominal pain, hemorrhagic diarrhea, soft feces, and hepatomegaly (enlarged liver).[9]
Odevixibat was approved for medical use in the United States and in the European Union in July 2021.[5][6][7][13][14] The U.S. Food and Drug Administration considers it to be a first-in-class medication.[15]
Medical uses
In the United States, odevixibat is indicated for the treatment of pruritus in people three months of age and older with progressive familial intrahepatic cholestasis.[5] In the European Union it is indicated in people six months of age and older.[6][7]
Mechanism of action
Odevixibat is a reversible inhibitor of the ileal sodium/bile acid co-transporter. This transporter is responsible for reabsorption of the majority of bile acids in the distal ileum.[16] The reduced absorption of the bile acids in the distal ileum compounds and leads to a decrease in stimulation of FXR (farnesoid X receptor), decreasing the inhibition of bile acid synthesis.[17]
Odevixibat works as a reversible, selective, small molecule inhibitor of the ileal bile acid transporter (IBAT).[9][11]
Pharmacokinetics
Odevixibat is > 99% protein-bound in vitro.[17] A dose of odevixibat that is 7.2 mg reaches a cmax concentration of 0.47 ng/mL with an AUC (0-24h) of 2.19 h*ng/mL.[17] Adult and pediatric patients given the therapeautic dose of odevixibat did not display plasma concentrations of the drug.[14] Odevixibat is eliminated majorly unchanged.[17] Odevixibat has an average half-life of 2.36 hours.[17]
The peak plasma time ranges from 1 to 5 hours after a single 7.2 mg dose in healthy adults. In healthy adults receiving a single 7.2 mg dose, the peak plasma concentration is 0.47 ng/mL, and the area under the concentration-time curve (AUC) is 2.19 ng·hr/mL. The plasma concentration of odevixibat in patients aged 6 months to 17 years ranges from 0.06 to 0.72 ng/mL. With once-daily dosing, there is no accumulation of odevixibat.[18]
Odevixibat is metabolized through a process called mono-hydroxylation.The drug is primarily eliminated through the feces (97% unchanged), with a minimal amount excreted in the urine (0.002%).[19]
Consuming a high-fat meal (800-1000 calories with approximately 50% of the total caloric content from fat) the peak plasma concentration is decreased by 72%, the AUC by 62%, and delays the peak plasma time by 3 to 4.5 hours. However, the impact of food on systemic exposures to odevixibat is not clinically significant.[20]
Contraindications
Odevixibat cannot be given to a child on a liquid diet.[17]
Adverse effects
Common side effects of odevixibat include diarrhea, stomach pain, vomiting, liver test abnormalities, abnormal liquid function tests, and a deficiency in vitamins A, D, E and K.[21][17]
Pregnancy and lactation
There are no enough human data on odevixibat use during pregnancy to build a drug-associated risk of major birth defects, miscarriage, or adverse developmental outcomes.[21]
There are no data on the presence of odevixibat in human milk, and how it affects milk production and breastfed babies.[21]
History
Preclinical studies and early clinical trials were conducted to evaluate the safety and efficacy of odevixibat, to establish the appropriate dosage, assess its mechanism of action, and evaluate its effects on bile acid levels and symptoms in people with progressive familial intrahepatic cholestasis. A 24-week clinical trial, played a role in demonstrating the effectiveness and safety of odevixibat in treating pruritus in children with progressive familial intrahepatic cholestasis.[19]
The US Food and Drug Administration (FDA) granted the application for odevixibat orphan drug designation.[15] The FDA classified odevixibat as an orphan drug for the rare conditions of Alagille syndrome, biliary atresia, and primary biliary cholangitis.[21]
Odevixibat was granted its initial approval in July 2021, in the European Union for the treatment of progressive familial intrahepatic cholestasis in people aged six months and older. In July 2021, it received approval in the United States for the treatment of pruritus (itching) in people aged three months and older with progressive familial intrahepatic cholestasis.[22]
Society and culture
Legal status
In May 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) recommended granting a marketing authorization in the European Union for odevixibat for the treatment of progressive familial intrahepatic cholestasis in people aged six months or older.[9][23] It was authorized for medical use in the European Union in July 2021.[6][7]
In July 2024, the CHMP adopted a positive opinion, recommending the granting of a marketing authorization under exceptional circumstances for the medicinal product Kayfanda, intended for the treatment of cholestatic pruritus in people with Alagille syndrome aged six months or older.[8][24] The applicant for this medicinal product is Ipsen Pharma.[8] Kayfanda was authorized for medical use in the European Union in September 2024.[8]
Research
A phase III randomized control trial showed odevixibat reduced pruritis and serum bile acids in children with progressive familial intrahepatic cholestasis.[25]
References
- ^ "Details for: Bylvay". Health Canada. 30 October 2023. Retrieved 3 March 2024.
- ^ "Notice: Multiple additions to the Prescription Drug List (PDL) [2023-12-22]". Health Canada. 22 December 2023. Retrieved 3 January 2024.
- ^ "Regulatory Decision Summary for Bylvay". Drug and Health Products Portal. 23 October 2023. Retrieved 2 April 2024.
- ^ "Summary Basis of Decision for Bylvay". Drug and Health Products Portal. 1 September 2012. Retrieved 9 May 2024.
- ^ a b c d e "Bylvay- odevixibat capsule, coated pellets". DailyMed. U.S. National Library of Medicine. Archived from the original on 29 July 2021. Retrieved 28 July 2021.
- ^ a b c d "Bylvay EPAR". European Medicines Agency (EMA). 20 April 2021. Archived from the original on 29 July 2021. Retrieved 28 July 2021.
- ^ a b c d "Bylvay". Union Register of medicinal products. Archived from the original on 24 July 2021. Retrieved 23 July 2021.
- ^ a b c d "Kayfanda EPAR". European Medicines Agency (EMA). 25 July 2024. Retrieved 25 July 2024. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ a b c d e "First treatment for rare liver disease". European Medicines Agency (EMA) (Press release). 21 May 2021. Retrieved 21 May 2021. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Odevixibat". Albireo Pharma. Archived from the original on 22 May 2021. Retrieved 21 May 2021.
- ^ a b Karpen SJ, Kelly D, Mack C, Stein P (September 2020). "Ileal bile acid transporter inhibition as an anticholestatic therapeutic target in biliary atresia and other cholestatic disorders". Hepatology International. 14 (5): 677–689. doi:10.1007/s12072-020-10070-w. PMID 32653991. S2CID 220481607.
- ^ Deeks ED (October 2021). "Odevixibat: First Approval". Drugs. 81 (15): 1781–1786. doi:10.1007/s40265-021-01594-y. PMC 8550539. PMID 34499340.
- ^ "Odevixibat: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 27 September 2021. Retrieved 23 July 2021.
- ^ a b "Albireo Announces FDA Approval of Bylvay (odevixibat), the First Drug Treatment for Patients With Progressive Familial Intrahepatic Cholestasis (PFIC)". Albireo Pharma (Press release). 20 July 2021. Retrieved 23 July 2021 – via GlobeNewswire.
- ^ a b Advancing Health Through Innovation: New Drug Therapy Approvals 2021 (PDF). U.S. Food and Drug Administration (FDA) (Report). 13 May 2022. Archived from the original on 6 December 2022. Retrieved 22 January 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Odevixibat". go.drugbank.com. Archived from the original on 19 September 2021. Retrieved 13 June 2022.
- ^ a b c d e f g "Odevixibat Monograph for Professionals". Drugs.com. 30 August 2022. Archived from the original on 15 December 2022. Retrieved 6 July 2023.
- ^ Yu S, Wang D, Yu J, Yin Y, Xie S, Cheng Q, et al. (May 2021). "Plasma or serum, which is the better choice for the measurement of metanephrines?". Scandinavian Journal of Clinical and Laboratory Investigation. 81 (3): 250–253. doi:10.1080/00365513.2021.1904280. PMID 33787416. S2CID 232430232. Archived from the original on 7 July 2023. Retrieved 7 July 2023.
- ^ a b Ray K (September 2022). "Positive phase III results for odevixibat for progressive familial intrahepatic cholestasis". Nature Reviews. Gastroenterology & Hepatology. 19 (9): 556. doi:10.1038/s41575-022-00667-x. PMID 35879356. S2CID 251021808.
- ^ Taylor L, Gidal B, Blakey G, Tayo B, Morrison G (November 2018). "A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects". CNS Drugs. 32 (11): 1053–1067. doi:10.1007/s40263-018-0578-5. PMC 6223703. PMID 30374683. Archived from the original on 7 July 2023. Retrieved 7 July 2023.
- ^ a b c d Porwal M, Kumar A, Rastogi V, Maheshwari KK, Verma A (March 2023). "Odevixibat: A Review of a Bioactive Compound for the Treatment of Pruritus Approved by the FDA". Current Drug Research Reviews. 15: 32–42. doi:10.2174/2589977515666230308125238. PMID 36892028. S2CID 257426837.
- ^ Deeks ED (October 2021). "Odevixibat: First Approval". Drugs. 81 (15): 1781–1786. doi:10.1007/s40265-021-01594-y. PMC 8550539. PMID 34499340. Archived from the original on 7 July 2023. Retrieved 7 July 2023.
- ^ "Bylvay: Pending EC decision". European Medicines Agency (EMA). 19 May 2021. Archived from the original on 21 May 2021. Retrieved 21 May 2021.
- ^ "Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 22-25 July 2024". European Medicines Agency (EMA) (Press release). 25 July 2024. Retrieved 29 July 2024.
- ^ Thompson RJ, Arnell H, Artan R, Baumann U, Calvo PL, Czubkowski P, et al. (September 2022). "Odevixibat treatment in progressive familial intrahepatic cholestasis: a randomised, placebo-controlled, phase 3 trial". The Lancet. Gastroenterology & Hepatology. 7 (9): 830–842. doi:10.1016/S2468-1253(22)00093-0. PMID 35780807. S2CID 250229742.
External links
- Clinical trial number NCT03566238 for "This Study Will Investigate the Efficacy and Safety of A4250 in Children With PFIC 1 or 2 (PEDFIC 1)" at ClinicalTrials.gov
