Nitrene

In chemistry, a nitrene or imene (R−:Ṅ·) is the nitrogen analogue of a carbene. The nitrogen atom is uncharged and monovalent,[1] so it has only 6 electrons in its valence level—two covalent bonded and four non-bonded electrons. It is therefore considered an electrophile due to the unsatisfied octet. A nitrene is a reactive intermediate and is involved in many chemical reactions.[2][3] The simplest nitrene, HN, is called imidogen, and that term is sometimes used as a synonym for the nitrene class.[4]
Electron configuration
In the simplest case, the linear N–H molecule (imidogen) has its nitrogen atom sp hybridized, with two of its four non-bonded electrons as a lone pair in an sp orbital and the other two occupying a degenerate pair of p orbitals. The electron configuration is consistent with Hund's rule: the low energy form is a triplet with one electron in each of the p orbitals and the high energy form is the singlet with an electron pair filling one p orbital and the other p orbital vacant.[5]
As with carbenes, a strong correlation exists between the spin density on the nitrogen atom which can be calculated in silico and the zero-field splitting parameter D which can be derived experimentally from electron spin resonance.[6] Small nitrenes such as NH or CF3N have D values around 1.8 cm−1 with spin densities close to a maximum value of 2. At the lower end of the scale are molecules with low D (< 0.4) values and spin density of 1.2 to 1.4 such as 9-anthrylnitrene and 9-phenanthrylnitrene.
Formation
Because nitrenes are so reactive, they are rarely isolated. Instead, they are formed as reactive intermediates during a reaction. There are two common ways to generate nitrenes:
- From azides by thermolysis or photolysis, with expulsion of nitrogen gas. This method is analogous to the formation of carbenes from diazo compounds.
- From isocyanates, with expulsion of carbon monoxide. This method is analogous to the formation of carbenes from ketenes.
Since formation of the nitrene typically starts from a diamagnetic precursor, the direct chemical product is a singlet nitrene, which then relaxes to its ground state triplet state. As has been shown for phenylazide as a model system, the direct photoproduct of photochemical-induced N2 loss can either be the singlet or triplet nitrene.[7][8][9] By using a triplet sensitizer, the triplet nitrene can also be formed without initial formation of the singlet nitrene.[10]
Isolated Nitrenes
Although highly reactive, some nitrenes could be isolated and characterized recently.
In 2019, a triplet nitrene was isolated by Betley and Lancaster, stabilized by coordination to a copper center in a bulky ligand.[11] Later on, Schneider and coworkers characterized Pd and Pt triplet metallonitrenes, where the organic residue is replaced by a metal.[12][13][14] In 2024, the groups of Beckmann, Ye and Tan reported the isolation and characterization of organic triplet nitrenes, which are protected from chemical reactivity by an extremely bulky ligand.[15][16]
Reactions
Nitrene reactions include:
- Nitrene C–H insertion. A nitrene can easily insert into a carbon to hydrogen covalent bond yielding an amine or amide. A singlet nitrene reacts with retention of configuration. In one study[17] a nitrene, formed by oxidation of a carbamate with potassium persulfate, gives an insertion reaction into the palladium to nitrogen bond of the reaction product of palladium(II) acetate with 2-phenylpyridine to methyl N-(2-pyridylphenyl)carbamate in a cascade reaction:
- A nitrene intermediate is suspected in this C–H insertion involving an oxime, acetic anhydride leading to an isoindole:[18]
- Nitrene cycloaddition. With alkenes, nitrenes react to form aziridines, very often with nitrenoid precursors such as nosyl- or tosyl-substituted [N-(phenylsulfonyl)imino]phenyliodinane (PhI=NNs or PhI=NTs respectively)) but the reaction is known to work directly with the sulfonamide in presence of a transition metal based catalyst such as copper, palladium, or gold:[19][20][21][22][23]
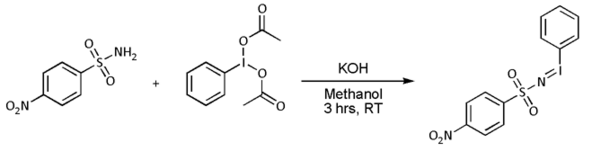
- In most cases, however, [N-(p-nitrophenylsulfonyl)imino]phenyliodinane (PhI=NNs) is prepared separately as follows:
- Nitrene transfer takes place next:
- In this particular reaction both the cis-stilbene illustrated and the trans form (not depicted) result in the same trans-aziridine product, suggesting a two-step reaction mechanism. The energy difference between triplet and singlet nitrenes can be very small in some cases, allowing interconversion at room temperature. Triplet nitrenes are thermodynamically more stable but react stepwise allowing free rotation and thus producing a mixture of stereochemistry.[24]
- Arylnitrene ring-expansion and ring-contraction: Aryl nitrenes show ring expansion to 7-membered ring cumulenes, ring opening reactions and nitrile formations many times in complex reaction paths. For instance the azide 2 in the scheme below[6] trapped in an argon matrix at 20 K on photolysis expels nitrogen to the triplet nitrene 4 (observed experimentally with ESR and ultraviolet-visible spectroscopy) which is in equilibrium with the ring-expansion product 6.
- The nitrene ultimately converts to the ring-opened nitrile 5 through the diradical intermediate 7. In a high-temperature reaction, FVT at 500–600 °C also yields the nitrile 5 in 65% yield.[25]
Nitreno radicals
For several compounds containing both a nitrene group and a free radical group an ESR high-spin quartet has been recorded (matrix, cryogenic temperatures). One of these has an amine oxide radical group incorporated,[26] another system has a carbon radical group.[27]
In this system one of the nitrogen unpaired electrons is delocalized in the aromatic ring making the compound a σ–σ–π triradical. A carbene nitrogen radical (imidyl radical) resonance structure makes a contribution to the total electronic picture.
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "nitrenes". doi:10.1351/goldbook.N04145
- ^ Lwowski, W., ed. (1970). Nitrenes. New York: Interscience.
- ^ Wentrup, C. (1984). Reactive Intermediates. New York: Wiley.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "imidogens". doi:10.1351/goldbook.I02951
- ^ Vyas, Shubham; Winter, Arthur H.; Hadad, Christopher M. (2013), "Theory and Computation in the Study of Nitrenes and their Excited-State Photoprecursors", Nitrenes and Nitrenium Ions, John Wiley & Sons, Ltd, pp. 33–76, doi:10.1002/9781118560907.ch2, ISBN 978-1-118-56090-7, retrieved 20 December 2024
- ^ a b Kvaskoff, David; Bednarek, Paweł; George, Lisa; Waich, Kerstin; Wentrup, Curt (2006). "Nitrenes, Diradicals, and Ylides. Ring Expansion and Ring Opening in 2-Quinazolylnitrenes". J. Org. Chem. 71 (11): 4049–4058. doi:10.1021/jo052541i. PMID 16709043.
- ^ Gritsan, N. P.; Platz, M. S. (1 September 2006). "Kinetics, Spectroscopy, and Computational Chemistry of Arylnitrenes". Chemical Reviews. 106 (9): 3844–3867. doi:10.1021/cr040055+. ISSN 0009-2665.
- ^ Soto, Juan; Otero, Juan C. (24 October 2019). "Conservation of El-Sayed's Rules in the Photolysis of Phenyl Azide: Two Independent Decomposition Doorways for Alternate Direct Formation of Triplet and Singlet Phenylnitrene". The Journal of Physical Chemistry A. 123 (42): 9053–9060. doi:10.1021/acs.jpca.9b06915. ISSN 1089-5639.
- ^ Domenianni, Luis I.; Bauer, Markus; Schmidt-Räntsch, Till; Lindner, Jörg; Schneider, Sven; Vöhringer, Peter (2023). "Photoinduced Metallonitrene Formation by N2 Elimination from Azide Diradical Ligands". Angewandte Chemie International Edition. 62 (42): e202309618. doi:10.1002/anie.202309618. ISSN 1521-3773.
- ^ Murthy, Rajesh S.; Muthukrishnan, Sivaramakrishnan; Rajam, Sridhar; Mandel, Sarah M.; Ault, Bruce S.; Gudmundsdottir, Anna D. (25 January 2009). "Triplet-sensitized photolysis of alkoxycarbonyl azides in solution and matrices". Journal of Photochemistry and Photobiology A: Chemistry. 201 (2): 157–167. doi:10.1016/j.jphotochem.2008.10.015. ISSN 1010-6030.
- ^ Carsch, K. M.; DiMucci, I. M.; Iovan, D. A.; Li, A.; Zheng, S.-L.; Titus, C. J.; Lee, S. J.; Irwin, K. D.; Nordlund, D.; Lancaster, K. M.; Betley, T. A. (2019). "Synthesis of a Copper-Supported Triplet Nitrene Complex Pertinent to Copper-Catalyzed Amination". Science. 365 (6458): 1138–1143. Bibcode:2019Sci...365.1138C. doi:10.1126/science.aax4423. PMC 7256962. PMID 31515388.
- ^ Sun, Jian; Abbenseth, Josh; Verplancke, Hendrik; Diefenbach, Martin; de Bruin, Bas; Hunger, David; Würtele, Christian; van Slageren, Joris; Holthausen, Max C.; Schneider, Sven (November 2020). "A platinum(ii) metallonitrene with a triplet ground state". Nature Chemistry. 12 (11): 1054–1059. doi:10.1038/s41557-020-0522-4. hdl:11245.1/1d9bd22a-92be-40ac-9b3a-e6dc7df2afc9. ISSN 1755-4349.
- ^ Schmidt-Räntsch, Till; Verplancke, Hendrik; Lienert, Jonas N.; Demeshko, Serhiy; Otte, Matthias; Van Trieste III, Gerard P.; Reid, Kaleb A.; Reibenspies, Joseph H.; Powers, David C.; Holthausen, Max C.; Schneider, Sven (2022). "Nitrogen Atom Transfer Catalysis by Metallonitrene C−H Insertion: Photocatalytic Amidation of Aldehydes". Angewandte Chemie International Edition. 61 (9): e202115626. doi:10.1002/anie.202115626. ISSN 1521-3773. PMC 9305406. PMID 34905281.
- ^ Schmidt-Räntsch, Till; Verplancke, Hendrik; Kehl, Annemarie; Sun, Jian; Bennati, Marina; Holthausen, Max C.; Schneider, Sven (23 September 2024). "C═C Dissociative Imination of Styrenes by a Photogenerated Metallonitrene". JACS Au. 4 (9): 3421–3426. doi:10.1021/jacsau.4c00571. PMC 11423323. PMID 39328761.
- ^ Janssen, Marvin; Frederichs, Thomas; Olaru, Marian; Lork, Enno; Hupf, Emanuel; Beckmann, Jens (19 July 2024). "Synthesis of a stable crystalline nitrene". Science. 385 (6706): 318–321. doi:10.1126/science.adp4963.
- ^ Wang, Dongmin; Chen, Wang; Chen, Haonan; Chen, Yizhen; Ye, Shengfa; Tan, Gengwen (19 November 2024). "Isolation and characterization of a triplet nitrene". Nature Chemistry: 1–6. doi:10.1038/s41557-024-01669-9. ISSN 1755-4349.
- ^ Thu, Hung-Yat; Yu, Wing-Yiu; Che, Chi-Ming (2006). "Intermolecular Amidation of Unactivated sp2 and sp3 C–H Bonds via Palladium-Catalyzed Cascade C–H Activation/Nitrene Insertion". J. Am. Chem. Soc. 128 (28): 9048–9049. doi:10.1021/ja062856v. PMID 16834374.
- ^ Savarin, Cécile G.; Grisé, Christiane; Murry, Jerry A.; Reamer, Robert A.; Hughes, David L. (2007). "Novel Intramolecular Reactivity of Oximes: Synthesis of Cyclic and Spiro-Fused Imines". Org. Lett. 9 (6): 981–983. doi:10.1021/ol0630043. PMID 17319674.
- ^ Li, Zigang; Ding, Xiangyu; He, Chuan (2006). "Nitrene Transfer Reactions Catalyzed by Gold Complexes". J. Org. Chem. 71 (16): 5876–5880. doi:10.1021/jo060016t. PMID 16872166. S2CID 43641348.
- ^ Evans, David A.; Faul, Margaret M.; Bilodeau, Mark T. (1994). "Development of the Copper-Catalyzed Olefin Aziridination Reaction". J. Am. Chem. Soc. 116 (7): 2742–2753. doi:10.1021/ja00086a007. S2CID 55554519.
- ^ Brandt, Peter; Sodergren, Mikael J.; Andersson, Pher G.; Norrby, Per-Ola (2000). "Mechanistic Studies of Copper-Catalyzed Alkene Aziridination". J. Am. Chem. Soc. 122 (33): 8013–8020. doi:10.1021/ja993246g. S2CID 98310736.
- ^ Watson, Iain D. G.; Yu, Lily; Yudi, Andrei K. (2006). "Advances in Nitrogen Transfer Reactions Involving Aziridines". Acc. Chem. Res. 39 (3): 194–206. doi:10.1021/ar050038m. PMID 16548508.
- ^ Reactants cis-stilbene or trans-stilbene, nitrene precursor p-nitrosulfonamide or nosylamine which is oxidized by iodosobenzene diacetate. The gold catalyst is based on a terpyridine tridentate ligand.
- ^ Yudin, Andrei K., ed. (2007). Aziridines and Epoxides in Organic Synthesis. p. 120. ISBN 978-3-527-31213-9.
- ^ The quinazoline is prepared from the corresponding bromide and sodium azide. The azide is in equilibrium with the tetrazole 3.
- ^ Lahti, Paul M.; Esat, Burak; Liao, Yi; Serwinski, Paul; Lan, Jiang; Walton, Richard (30 May 2001). "Heterospin organic molecules: nitrene–radical linkages". Polyhedron. 20 (11–14): 1647–1652. doi:10.1016/S0277-5387(01)00667-2.
- ^ Sander, Wolfram; Grote, Dirk; Kossmann, Simone; Neese, Frank (2008). "2,3,5,6-Tetrafluorophenylnitren-4-yl: Electron Paramagnetic Resonance Spectroscopic Characterization of a Quartet-Ground-State Nitreno Radical". J. Am. Chem. Soc. 130 (13): 4396–4403. doi:10.1021/ja078171s. PMID 18327939.