Mitozolomide
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.079.921 |
| Chemical and physical data | |
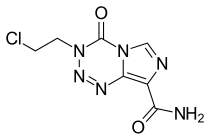
| Formula | C7H7ClN6O2 |
| Molar mass | 242.62 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Mitozolomide (INN) is an antineoplastic. It is an imidazotetrazine derivative.
Development of mitozolomide was discontinued during Phase II clinical trials after it was found to cause severe and unpredictable bone marrow suppression.[1] Temozolomide, which has been in clinical use since 1999, is a less toxic analogue of mitozolomide.[2]
References
- ^ Fairbairn LJ, Chinnasamy N, Lashford LS, Chinnasamy D, Rafferty JA (February 2000). "Enhancing hemopoietic drug resistance: a rationale for reconsidering the clinical use of mitozolomide" (PDF). Cancer Gene Ther. 7 (2): 233–9. doi:10.1038/sj.cgt.7700120. PMID 10770631. S2CID 2597751.
- ^ Newlands ES, Blackledge GR, Slack JA, et al. (February 1992). "Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856)". Br J Cancer. 65 (2): 287–91. doi:10.1038/bjc.1992.57. PMC 1977719. PMID 1739631.
