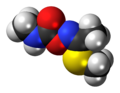
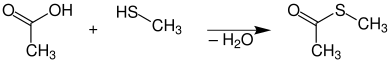Methomyl
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name (E,Z)-methyl N-{[(methylamino)carbonyl]oxy}ethanimidothioate | |||
| Other names Lannate, Mesomile, Methomex, Nudrin | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.037.089 | ||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C5H10N2O2S | |||
| Molar mass | 162.20 | ||
| Appearance | White crystalline solid[2] | ||
| Odor | Slight, sulfur-like[2] | ||
| Density | 1.2946 g/cm3 | ||
| Melting point | 78 to 79 °C (172 to 174 °F; 351 to 352 K) | ||
| 58 g/L | |||
| Vapor pressure | 0.00005 mmHg (25°C)[2] | ||
| Hazards | |||
| Flash point | Noncombustible[2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
none[2] | ||
REL (Recommended) |
TWA 2.5 mg/m3[2] | ||
IDLH (Immediate danger) |
N.D.[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Methomyl is a carbamate insecticide introduced in 1966. It is highly toxic to humans, livestock, pets, and wildlife.[3] The EU imposed a pesticide residue limit of 0,01 mg/kg for all fruit and vegetables.[4]
Methomyl is a common active ingredient in commercial fly bait, for which the label instructions in the United States warn that "It is a violation of Federal Law to use this product in a manner inconsistent with its labeling." "Off-label" uses and other uses not specifically targeted at problem insects are illegal, dangerous, and ill-advised.[5][6]
Use
Methomyl is a broad-spectrum insecticide that is used to kill insect pests.[7] Methomyl is registered for commercial/professional use under certain conditions on sites including field, vegetable, and orchard crops; turf (sod farms only); livestock quarters; commercial premises; and refuse containers. Products containing 1% Methomyl are available to the general public for retail sale, but more potent formulations are classified as restricted-use pesticides: not registered for homeowner or non-professional application.[7] However, Heliothis virescens developed a resistance to methomyl within 5 years.[8] Other species like Helicoverpa assulta also developed resistance after exposure.[9]
Toxicity
In acute toxicity testing, methomyl is placed in EPA Toxicity Category I (the highest toxicity category out of four) via the oral route and in eye irritation studies.[7] It is in lower Toxicity Categories for inhalation (Category II), acute dermal effects (Category III), and acute skin irritation (Category IV). Methomyl is not likely to be a carcinogen (EPA carcinogen Category E).[7]
Ecotoxicity
Methomyl has low persistence in the soil environment, with a reported half-life of approximately 14 days.[10] Because of its high solubility in water, and low affinity for soil binding methomyl may have potential for groundwater contamination.[7][11] The estimated aqueous half-life for the insecticide is 6 days in surface water and over 25 weeks in groundwater.[11]
Synthesis
First prepare thioester:
Second prepare oxime from thioester:
Third prepare product from methyl isocyanate and the finished oxime:
Trade names
Common names for methomyl include metomil and mesomile. Trade names include Acinate, Agrinate, DuPont 1179, Flytek, Kipsin, Lannate, Lanox, Memilene, Methavin, Methomex, Nudrin, NuBait, Pillarmate and SD 14999 [11]
References
- ^ Merck Index, 11th Edition, 5905
- ^ a b c d e f g NIOSH Pocket Guide to Chemical Hazards. "#0387". National Institute for Occupational Safety and Health (NIOSH).
- ^ Methomyl at Extension Toxicology Network
- ^ "EU pesticides database".
- ^ Conservation Warden Warns: Fly bait to control wild animals – illegal and a bad idea (Wisconsin Department of Natural Resources)
- ^ "Farm stores promoted poisoning raccoons, state chemist says". Archived from the original on 2015-09-24. Retrieved 2012-08-09.
- ^ a b c d e EPA R.E.D. FACTS - Methomyl (PDF) (Technical report). U. S. Environmental Protection Agency. December 1998. EPA-738-F-98-019.
- ^ Blanco, Carlos (2012). "Heliothis virescens and Bt cotton in the United States". GM Crops & Food: Biotechnology in Agriculture and the Food Chain. 3 (3): 201–212. doi:10.4161/gmcr.21439. PMID 22892654.
- ^ Wang, Kai-Yun; Zhang, Yong; Wang, Hong-Yan; Xia, Xiao-Ming; Liu, Tong-Xian (2010-01-01). "Influence of three diets on susceptibility of selected insecticides and activities of detoxification esterases of Helicoverpa assulta (Lepidoptera: Noctuidae)". Pesticide Biochemistry and Physiology. 96 (1): 51–55. doi:10.1016/j.pestbp.2009.09.003.
- ^ Howard, P. H. (1991). Handbook of Environmental Fate and Exposure Data for Organic Chemicals: Pesticides. Chelsea, MI: Lewis Publishers. pp. 3–15.
- ^ a b c "Extoxnet Pip - Methomyl".
External links
- Methomyl in the Pesticide Properties DataBase (PPDB)