Metepa
| |
| Names | |
|---|---|
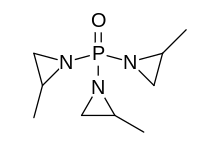
| IUPAC name 1-[Bis(2-methyl-1-aziridinyl)phosphoryl]-2-methylaziridine | |
| Preferred IUPAC name 1,1′,1″-Phosphoryltris(2-methylaziridine) | |
| Other names Methaphoxide Metapoxide Methyl aphoxide METEPA Trimethylaziridinylphosphine oxide MAPO Tris(1,2-propylene)phosphoramide Tris(2-methyl-1-aziridinyl)phosphine oxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.000.296 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties[1] | |
| C9H18N3OP | |
| Molar mass | 215.237 g·mol−1 |
| Appearance | Colorless liquid |
| Boiling point | 90 to 92 °C (194 to 198 °F; 363 to 365 K) (0.15-0.3 mmHg) |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
136 mg/kg (male rat, oral)[1] 213 mg/kg (female rat, oral)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Metepa is a chemosterilant, with the capability to restrict ovarian development.[2] Metepa can also result in carcinogenesis, in particular the formation of teratomas.[3] It a minor ingredient in certain solid rocket propellants.[4]
References
- ^ a b c Merck Index, 12th Edition, 5998
- ^ Morgan, Philip B; Labrecque, G. C (1964). "Effect of Tepa and Metepa on Ovarian Development of House Flies". Journal of Economic Entomology. 57 (6): 896–899. doi:10.1093/jee/57.6.896.
- ^ Gaines, T. B; Kimbrough, R. D (1966). "The sterilizing, carcinogenic and teratogenic effects of metepa in rats". Bulletin of the World Health Organization. 34 (2): 317–20. PMC 2475925. PMID 5296141.
- ^ "Ababil-100/Al Fat'h". GlobalSecurity.org. Archived from the original on 15 April 2019.
