Malonic aciduria
| Malonic aciduria | |
|---|---|
| Other names | Malonyl-CoA decarboxylase deficiency, classic CMAMMA |
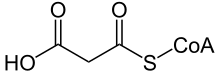 | |
| Malonyl-CoA | |
Malonic aciduria or malonyl-CoA decarboxylase deficiency (MCD) is an autosomal-recessive[1] metabolic disorder caused by a genetic mutation that disrupts the activity of Malonyl-CoA decarboxylase. This enzyme breaks down Malonyl-CoA (a fatty acid precursor and a fatty acid oxidation blocker) into acetyl-CoA and carbon dioxide.
Signs and symptoms
The signs and symptoms of this disorder typically appear in early childhood. Almost all affected children have delayed development. Additional signs and symptoms can include weak muscle tone (hypotonia), seizures, diarrhea, vomiting, and low blood sugar (hypoglycemia). A heart condition called cardiomyopathy, which weakens and enlarges the heart muscle, is another common feature of malonic aciduria.
Genetics

Malonic aciduria is caused by mutations in the MLYCD gene, located on chromosome 16q23.3.[2] The gene encodes the enzyme malonyl-CoA decarboxylase. Within cells, this enzyme helps regulate the formation and breakdown of a certain group of fats called fatty acids.
Many tissues, including heart muscle, use fatty acids as a major source of energy. Mutations in the MLYCD gene reduce or eliminate the function of malonyl-CoA decarboxylase. A lack of this enzyme disrupts the normal balance of fatty acid formation and breakdown. As a result, fatty acids cannot be converted to energy, which can lead to characteristic features of this disorder, such as low blood sugar and cardiomyopathy. By-products of fatty acid processing build up in tissues, which also contributes to the signs and symptoms of malonic aciduria.
Malonic aciduria is inherited in an autosomal recessive pattern.[1] This means that the defective gene is located on an autosome (chromosome 16 is an autosome), and two copies of the defective gene - one inherited from each parent - are required to be born with the disorder. The parents of a child with an autosomal recessive disorder both carry one copy of the defective gene, but are usually not affected by the disorder.
Malonic aciduria is extremely rare, evidence suggests that it is caused by the abnormality in the protein transcription regulation.[3] Looking at the molecular basis, two distinct homozygous mutations are found to cause malonic aciduria in human. The first mutation is the transversion of gene from C to G causing a premature stop signal in the protein. The second mutation is a base pair insertion in the mature RNA that will eventually result in the protein truncation.[4]
A research has also confirmed that the homozygous mutation which eventually leads to malonic aciduria is caused by the isodisomy of maternal UPD. This indicates that such disease is likely to be inherited from mother’s gene profile, not from paternal source.[5]
Prevalence
According to Orphanet (2006), the prevalence is less than 1 in 1,000,000.[6]
In 1984, malonic aciduria was described for the first time in a scientific study.[7]
By 1999, only seven cases of malonic aciduria had been reported in human in Australia; however, this deficiency predominately occurs during childhood. Patients from the seven reported cases of malonic aciduria have an age range between 4 days to 13 years, and they all have the common symptom of delayed neurological development.[4]
By 2006, 17 cases of malonic aciduria had been published in the literature worldwide, ranging in age from 8 days to 12 years.[3]
By 2017, less than 30 cases were known in the literature.[8]
Pathophysiology
Malonyl-CoA decarboxylase acts as a catalyst in the conversion of malonyl-CoA to acetyl-CoA and CO2.[9] Without the enzymatic activity of malonyl-CoA decarboxylase, cellular malonyl-CoA increases so dramatically that at the end it is instead broken down by an unspecific short-chain acyl-CoA hydrolase, which produces malonic acid and CoA. Malonic acid is a Krebs cycle inhibitor, preventing the cells to make ATP through oxidation. In this condition, the cells, to make ATP, are forced to increase glycolysis, which produces lactic acid as a by-product. The increase of lactic and malonic acid drastically lowers blood pH, and causes both lactic and malonic aciduria (acidic urine). It is also speculated that the excess of mitochondrial malonyl-CoA increases the methylmalonic acid level, which is due to an inhibitory effect on the methylmalonyl-CoA mutase.[10][11]
In the cytoplasm, malonyl-CoA acts as an inhibitor of the mitochondrial outer membrane enzyme carnitine palmitoyltransferase I (CPT1), which consequently inhibits fatty acid oxidation.[3] The inhibitory effect of cytoplastic malonyl-CoA on CPT1 varies, so it inhibits roughly 100-fold greater in cardiac and skeletal muscles than in the liver.[12]
Some common symptoms in malonic aciduria, such as cardiomyopathy and metabolic acidosis, are triggered by the high concentrations of malonyl-CoA in the cytoplasm.
Although we have not yet gained a clear understanding of the pathogenic mechanism of this deficiency, some researchers have suggested a brain-specific interaction between malonyl-CoA and CTP1 enzyme which may leads to unexplained symptoms of the malonic aciduria.[5]
Research has found that large amount of malonyl-CoA carboxylase are detached in the hypothalamus and cortex of the brain where high levels of lipogenic enzymes are found, indicating that malonyl-CoA decarboxylase plays a role in lipid synthesis in the brain.[3] Disturbed interaction between malonyl-CoA and CPT1 may also contributed to abnormal brain development.[3]
Malonyl-CoA decarboxylase plays an important role in the β-oxidation processes in both mitochondria and peroxisome.[4] Some other authors have also hypothesized that it is the Malonyl-CoA carboxylase deficiency induced inhibition of peroxisomal β-oxidation that contributes to the development delay.[4]
Diagnosis
Screening for elevated organic acids, especially malonic acid and methylmalonic acid and for high malonylcarnitine.[6] The diagnosis can then be confirmed by determining the enzyme activity of malonyl-coA decarboxylase in cultured skin fibroblasts.[6] Molecular genetic testing of the MLYCD gene may also be useful.[6]
Differential diagnosis
Combined malonic and methylmalonic aciduria (CMAMMA) & classic methylmalonic acidemia
By calculating the malonic acid to methylmalonic acid ratio in blood plasma, malonic aciduria can be clearly distinguished from combined malonic and methylmalonic aciduria (CMAMMA) and classic methylmalonic acidemia.[13] The latter applies for both, vitamin B12 responders and non-responders in methylmalonic acidemia.[13] In malonic aciduria, the results of the ratio are greater than 1, as the malonic acid value is higher than the methylmalonic acid value.[3] In CMAMMA, on the other hand, the result is less than 1 because the methylmalonic acid is higher than the malonic acid.[10]
Treatment
A research conducted in Netherlands has suggested that carnitine supplements and a low fat diet may help to reduce the level of malonic acid in our body.[5]
Note
As both malonic acid and methylmalonic acid levels are elevated in malonic aciduria, it used to be referred to as combined malonic and methylmalonic aciduria (CMAMMA). Although ACSF3 deficiency was not discovered until later, the term combined malonic and methylmalonic aciduria has now become established in medical databases for ACSF3 deficiency.[14][15]
See also
References
- ^ a b MacPhee, G. B.; Logan, R. W.; Mitchell, J. S.; Howells, D. W.; Tsotsis, E.; Thorburn, D. R. (October 1993). "Malonyl coenzyme a decarboxylase deficiency". Archives of Disease in Childhood. 69 (4): 433–436. doi:10.1136/adc.69.4.433. PMC 1029550. PMID 8259873.
- ^ Online Mendelian Inheritance in Man (OMIM): 606761
- ^ a b c d e f de Wit MC, de Coo IF, Verbeek E, Schot R, Schoonderwoerd GC, Duran M, de Klerk JB, Huijmans JG, Lequin MH, Verheijen FW, Mancini GM (February 2006). "Brain abnormalities in a case of malonyl-CoA decarboxylase deficiency". Molecular Genetics and Metabolism. 87 (2): 102–6. doi:10.1016/j.ymgme.2005.09.009. PMID 16275149.
- ^ a b c d FitzPatrick, DR; Hill, A; Tolmie, JL; Thorburn, DR; Christodoulou, J (August 1999). "The molecular basis of malonyl-CoA decarboxylase deficiency". American Journal of Human Genetics. 65 (2): 318–26. doi:10.1086/302492. PMC 1377930. PMID 10417274.
- ^ a b c Malvagia S, Papi L, Morrone A, Donati MA, Ciani F, Pasquini E, la Marca G, Scholte HR, Genuardi M, Zammarchi E (November 2007). "Fatal malonyl CoA decarboxylase deficiency due to maternal uniparental isodisomy of the telomeric end of chromosome 16". Annals of Human Genetics. 71 (Pt 6): 705–12. doi:10.1111/j.1469-1809.2007.00373.x. PMID 17535268. S2CID 35678278.
- ^ a b c d "Malonic aciduria". Orphanet. Retrieved 2024-04-20.
- ^ Brown, G. K.; Scholem, R. D.; Bankier, A.; Danks, D. M. (March 1984). "Malonyl coenzyme a decarboxylase deficiency". Journal of Inherited Metabolic Disease. 7 (1): 21–26. doi:10.1007/BF01805615. ISSN 0141-8955. PMID 6145813.
- ^ Ramaswamy, Mamatha; Skrinska, Victor Anthony; Abdoh, Ghassan; Mahmoud Ahmed, Laila; Mitri, Rola Fayez; Joshi, Ravi (March 2017). "A Rare Case of Malonic Aciduria Diagnosed by Newborn Screening in Qatar". International Journal of Neonatal Screening. 3 (1): 5. doi:10.3390/ijns3010005. ISSN 2409-515X.
- ^ Dewit, M; Decoo, I; Verbeek, E; Schot, R; Schoonderwoerd, G; Duran, M; Deklerk, J; Huijmans, J; Lequin, M; Verheijen, F (February 2006). "Brain abnormalities in a case of malonyl-CoA decarboxylase deficiency". Molecular Genetics and Metabolism. 87 (2): 102–106. doi:10.1016/j.ymgme.2005.09.009. PMID 16275149.
- ^ a b Alfares, A.; Nunez, L. D.; Al-Thihli, K.; Mitchell, J.; Melancon, S.; Anastasio, N.; Ha, K. C. H.; Majewski, J.; Rosenblatt, D. S.; Braverman, N. (2011-09-01). "Combined malonic and methylmalonic aciduria: exome sequencing reveals mutations in the ACSF3 gene in patients with a non-classic phenotype". Journal of Medical Genetics. 48 (9): 602–605. doi:10.1136/jmedgenet-2011-100230. ISSN 0022-2593. PMID 21785126.
- ^ Gregg, A. R.; Warman, A. W.; Thorburn, D. R.; O'Brien, W. E. (June 1998). "Combined malonic and methylmalonic aciduria with normal malonyl-coenzyme A decarboxylase activity: A case supporting multiple aetiologies". Journal of Inherited Metabolic Disease. 21 (4): 382–390. doi:10.1023/A:1005302607897. ISSN 0141-8955. PMID 9700595.
- ^ McGarry, J. Denis; Brown, Nicholas F. (February 1997). "The Mitochondrial Carnitine Palmitoyltransferase System — From Concept to Molecular Analysis". European Journal of Biochemistry. 244 (1): 1–14. doi:10.1111/j.1432-1033.1997.00001.x. ISSN 0014-2956. PMID 9063439.
- ^ a b de Sain-van der Velden, Monique G. M.; van der Ham, Maria; Jans, Judith J.; Visser, Gepke; Prinsen, Hubertus C. M. T.; Verhoeven-Duif, Nanda M.; van Gassen, Koen L. I.; van Hasselt, Peter M. (2016), Morava, Eva; Baumgartner, Matthias; Patterson, Marc; Rahman, Shamima (eds.), "A New Approach for Fast Metabolic Diagnostics in CMAMMA", JIMD Reports, Volume 30, vol. 30, Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 15–22, doi:10.1007/8904_2016_531, ISBN 978-3-662-53680-3, PMC 5110436, PMID 26915364
- ^ "COMBINED MALONIC AND METHYLMALONIC ACIDURIA". OMIM. Retrieved 2024-04-20.
- ^ "Combined malonic and methylmalonic acidemia". National Library of Medicine. Retrieved 2024-04-20.
External links
- Malonic aciduria at NLM Genetics Home Reference
