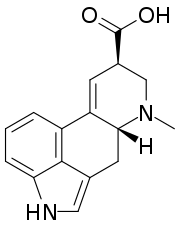
Lysergic acid
| |

| |
| Names | |
|---|---|
| IUPAC name 6-Methyl-9,10-didehydroergoline-8β-carboxylic acid | |
| Systematic IUPAC name (5R,8R)-7-Methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinoline-9-carboxylic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.302 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H16N2O2 | |
| Molar mass | 268.316 g·mol−1 |
| Melting point | 238 to 240 °C (460 to 464 °F; 511 to 513 K) |
| Acidity (pKa) | pKa1 = 7.80, pKa2 = 3.30 [1] |
| Legal status | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Lysergic acid, also known as D-lysergic acid and (+)-lysergic acid, is a precursor for a wide range of ergoline alkaloids that are produced by the ergot fungus and found in the seeds of Argyreia nervosa (Hawaiian baby woodrose), and Ipomoea species (morning glories, ololiuhqui, tlitliltzin).
Amides of lysergic acid, lysergamides, are widely used as pharmaceuticals and as psychedelic drugs, e.g. lysergic acid diethylamide (LSD). Lysergic acid is listed as a Table I precursor under the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances.[3]
Lysergic acid received its name as it was a product of the lysis of various ergot alkaloids.[4]
Synthesis
Laboratory
Lysergic acid is generally produced by hydrolysis[5] of natural lysergamides, but can also be synthesized in the laboratory by a complex total synthesis, for example by Robert Burns Woodward's team in 1956.[6] An enantioselective total synthesis based on a palladium-catalyzed domino cyclization reaction has been described in 2011 by Fujii and Ohno.[7] Lysergic acid monohydrate crystallizes in very thin hexagonal leaflets when recrystallized from water. Lysergic acid monohydrate, when dried (140 °C at 2 mmHg or 270 Pa) forms anhydrous lysergic acid.
Biosynthesis
The biosynthetic route is based on the alkylation of the amino acid tryptophan with dimethylallyl diphosphate (isoprene derived from 3R-mevalonic acid) giving 4-dimethylallyl-L-tryptophan which is N-methylated with S-adenosyl-L-methionine. Oxidative ring closure followed by decarboxylation, reduction, cyclization, oxidation, and allylic isomerization yields D-(+)-lysergic acid.[4] The biosynthetic pathway has been reconsituted in transgenic baker's yeast.[8]
Isomers
Lysergic acid is a chiral compound with two stereocenters. The isomer with inverted configuration at carbon atom 8 close to the carboxyl group is called isolysergic acid. Inversion at carbon 5 close to the nitrogen atom leads to L-lysergic acid and L-isolysergic acid, respectively.

Legality
In the United States, Lysergic acid and Lysergic acid amide are Schedule III substances.[9]
See also
References
- ^ Brown, H. C.; et al. (1955). Braude, E. A.; Nachod, F. C. (eds.). Determination of Organic Structures by Physical Methods. New York, NY: Academic Press.
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-15.
- ^ "List of Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances Under International Control" (PDF). International Narcotics Control Board. Archived from the original (PDF) on 2008-02-27.
- ^ a b Schiff, P. L. (Oct 15, 2006). "Ergot and its alkaloids". Am. J. Pharm. Educ. 70 (5): 98. doi:10.5688/aj700598. PMC 1637017. PMID 17149427.
- ^ Martínková, L.; Kren, V.; Cvak, L.; Ovesná, M.; Prepechalová, I. (Nov 17, 2001). "Hydrolysis of lysergamide to lysergic acid by Rhodococcus equi A4". J. Biotechnol. 84 (1): 63–6. doi:10.1016/s0168-1656(00)00332-1. PMID 11035188.
- ^ Kornfeld, Edmund C.; Fornefeld, E. J.; Kline, G. Bruce; Mann, Marjorie J.; Morrison, Dwight E.; Jones, Reuben G.; Woodward, R. B. (1956). "The Total Synthesis of Lysergic Acid". J. Am. Chem. Soc. 78 (13): 3087–3114. doi:10.1021/ja01594a039.
- ^ Inuki, S.; Iwata, A.; Oishi, S.; Fujii, N.; Ohno, H. (2011). "Enantioselective Total Synthesis of (+)-Lysergic Acid, (+)-Lysergol, and (+)-Isolysergol by Palladium-Catalyzed Domino Cyclization of Allenes Bearing Amino and Bromoindolyl Groups". J. Org. Chem. 76 (7): 2072–2083. doi:10.1021/jo102388e. PMID 21361331.
- ^ Wong, Garrett; Lim, Li Rong; Tan, Yong Quan; Go, Maybelle Kho; Bell, David J.; Freemont, Paul S.; Yew, Wen Shan (2022). "Reconstituting the complete biosynthesis of D-lysergic acid in yeast". Nature Communications. 13 (1): 712. Bibcode:2022NatCo..13..712W. doi:10.1038/s41467-022-28386-6. PMC 8821704. PMID 35132076.
- ^ https://www.ecfr.gov/current/title-21/chapter-II/part-1308/subject-group-ECFRf62f8e189108c4d/section-1308.13
