In vitro maturation
In vitro maturation (IVM) is the technique of letting the contents of ovarian follicles and the oocytes inside mature in vitro. It can be offered to women with infertility problems, combined with In Vitro Fertilization (IVF), offering women pregnancy without ovarian stimulation.
History
In 1935, Pincus & Enzmann did the first experiment on immature rabbit oocyte, showing in vitro spontaneous maturation and fertilization.[1] They showed maturation occurs in isolation from normal follicular environment.[1] In 1965 Edwards then continued IVM studies in mouse, sheep, cow, pig, rhesus monkey and human.[2][3] By 1991, the first human pregnancy was recorded using IVM followed by IVF,[4] and in 1994 the first birth using IVM oocytes from polycystic ovarian syndrome patients was recorded highlighting that PCOS patient's oocytes are capable of maturation.[5]
Background
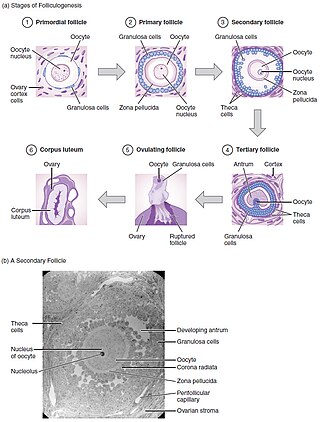
Oogenesis takes place during fetal life, in which primordial germ cells undergo mitosis until a few weeks prior to birth, forming oogonia. These then begin meiosis to form the oocyte within the primordial follicle.[6] This follicle consists of the oocyte surrounded by flattened pregranulosa cells. Babies are born with 1-2 million primordial follicles, and by puberty have around 300,000.[6] Of these primordial follicles, only around 400 mature oocytes are released and could be potentially fertilised, with the rest undergoing atresia.[7]
'Maturation' of an oocyte is the process by which an 'oocyte attains the competence to be fertilised and undergo embryogenesis'.[8]
Folliculogenesis is the mechanism by which the ovarian follicles mature. This can take many months in vivo and involves primordial follicle growth and differentiation.[8]
Primordial follicles containing the primary oocyte, arrested at prophase of meiosis I,[8] develop into primary follicle containing cuboidal granulosa cells. A secondary follicle is formed with a few granulosa cell layers, as well as a theca layer. Finally before ovulation, a tertiary follicle is formed containing a follicular-fluid filled antrum.[6] Of these small antral follicles, 1 will become dominant and ovulate (in monoovulatory species). During ovulation, the primary oocyte will resume meiosis in response to signals, arresting in metaphase meiosis II, ready for fertilization.[3] The dominant follicle contains the mature oocyte. Follicular development is directly under gonadotropins control, LH and FSH. These use cAMP as an intracellular second messenger, with growth factors and cytokines also influencing their development in vivo.[7]
Through in vitro maturation, folliculogenesis and latter parts of oogenesis are being mimicked outside of the ovaries- trying to recreate the conditions for these processes.
Techniques
If a follicle has reached the early tertiary or antral stage, IVM can be carried out.[9]
Firstly, the oocytes need to be obtained from the subject. The timing of this is dependent on the stage of the cycle the subject is in, which is usually monitored using ultrasonography.[10] If without the use of priming, oocytes are obtained when the largest follicles are around 10mm in size.[9]
In humans, this can be done with an aspiration needle, using ultrasound to allow accuracy. Depending on whether you are aspirating mature or immature follicles, the protocol differs slightly. In both procedures the aspiration pressure is reduced, but to varying degrees. Additionally, it is more important that the aspirate is filtered when retrieving immature follicles, as the follicles are much smaller and harder to see in the fluid extracted.[10]
Priming is the process by which the oocytes are primed with follicle-stimulating hormone (FSH) or human chorionic gonadotrophin (hCG) before retrieval. hCG is important in women with polycystic ovarian syndrome (PCOS). This results in an expanding or dispersed pattern of the cumulus oophorus around the egg cell, facilitating its identification within follicular fluid. This leads to improved maturation and quality of the oocytes.[7] However, the evidence of a clinical effect of hCG priming is still lacking.[11] When IVM was initially introduced, successful pregnancies were low, leading to the use of ovary priming.[10]
This technique is also used in sheep,[12] pigs[13] and other animals. See In animals.
Oocytes classification
Oocytes are classified depending on their condition, such as number of cumulus cells. The best oocytes are chosen to be matured in the hope of then being implanted using in vitro fertilisation techniques.[12]
Cultured in media
The oocytes are then cultured in media containing nutrients important for oocyte survival, such as gonadotrophins, growth factors and steroids.[10] These vary between clinics and research laboratories. McLaughlin et al. biopsied human ovarian tissue and achieved a 10% rate of maturation from unilaminar follicles into metaphase II by a multi-step culture system:[14]
- 8 days of culture in a serum-free medium
- 8 days of culture in a serum-free medium with activin A
- 4 days of culture on membranes with activin A and follicle-stimulating hormone (FSH).
In vitro fertilisation
Once the oocytes have sufficiently matured, they can then be fertilised in vitro, known as in vitro fertilisation (IVF). Techniques such as intracytoplasmic sperm injection (ICSI) can also be utilised to improve the chances of fertilisation being successful, which should be performed at least one hour (and optimally two to four hours) after the first polar body extrusion.[15] Out of in vitro matured oocytes, those fertilised with ICSI have a success rates of 60-80%, compared to IVF with success rates of 25-40%.[16]
A few live births have already been made by taking small early tertiary follicles, letting them mature in vitro and subsequently fertilizing them. However, for follicles that haven't reached the early tertiary stage, IVM is still under development. There are a lot of cellular changes in the oocyte and the rest of the cells in the follicle, which makes it very susceptible. Nevertheless, it is possible to let a primordial follicle mature to a secondary follicle outside the body by growing it in a slice of ovarian tissue. The subsequent maturity from secondary to early tertiary stage can then be supported in test-tubes.[16] It has been suggested that photoirradiation of granulosa cells and oocytes may facilitate IVM.[17]
Clinical applications
In vitro maturation is an assistive reproductive technique (ART) typically used in patients with fertility issues including polycystic ovary syndrome (PCOS), high antral follicle counts and ovarian hyper-responsiveness.[18][19] However, more recently IVM has also become widely utilised in areas such as fertility preservation in cancer patient who have undergone treatment involving gonadotoxic therapies.[18] There have been over 1000 live births recorded from mothers using IVM.[19]
Polycystic ovary syndrome
PCOS is a common disorder involving dysfunction of the endocrine system associated with female reproduction. PCOS involves discrepancies in the Hyphophyseal-pituitary-gonadal endocrine axis which can result in hormonal dysfunction, excess androgens (e.g. testosterone) and frequent anovulatory menstrual cycles.[20] Therefore, it is common for women suffering from PCOS to require assistance in order to conceive.[20][21][22] In these patients IVM can be used to mature oocytes and aid conception.[20][21] Few studies shows that substituting IVM in PCOS patients eliminates the risk of OHSS and lowers the cost of treatment. The same group conducted a retrospective analysis study to compare the treatment outcome of IVM with IVF in patients with PCOS.They have further concluded there was significant increase pregnancy rates, implantation rates and number of embryos transferred in IVM group.[23]
Alternative to ovarian hyperstimulation
The use of in vitro maturation in assisted reproduction has advantages over standard ART procedures. In typical IVF practice, controlled ovarian hyperstimulation is performed, which is where supraphysiological levels of gonadotropins are administered to the patient in order to hyperstimulate the antral follicles and hence induce oocyte maturation to metaphase II at a rate that is above normal physiological capabilities.[19] This practice can be disadvantageous in several ways: It is very costly, can become complicated and may also predispose to several undesirable side effects, such as ovarian hyperstimulation syndrome (OHSS).[19][21] Ovarian hyperstimulation can cause severe OHSS in up to 2% of cases. OHSS can have serious consequences, including respiratory problems, renal impairment and even stroke.[19] Patients with PCOS and younger women are at an increased risk of OHSS.[21] In these women, it may be even more beneficial to employ IVM rather than conventional IVF treatment.[19][21]
In IVM, immature oocytes are removed from the antral follicle of a woman and then are matured in vitro in a culture rich in gonadotrophins.[19] This hence negates (or significantly reduces) the need for gonadotrophin stimulation.[21]
IVM is not an entirely perfected technique. Pregnancy rates are lower in IVM than in standard IVF. There is also research required into whether or not babies born to mothers who have undergone IVM have any health concerns (e.g. developmental issues) later in life.[19]
Women with a personal or family history of an oestrogen associated thrombus, or of severe cardiovascular disease, may also benefit from IVM. This is because conventional IVF, with its hyperstimulation of the ovaries, has the potential to stimulate mass synthesis of oestrogen via the stimulation of granulosa cell oestrogen production.[19]
Ovarian tissue cryopreservation
Ovarian tissue cryopreservation can be used as a method of fertility preservation, such as before undergoing chemotherapy that can cause female infertility, or as a future resource in case the oocytes will stop functioning by advanced maternal age. Thus, ovarian tissue cryopreservation is an alternative to oocyte cryopreservation which requires a preceding controlled ovarian hyperstimulation. In vitro maturation allows oocytes from the ovarian tissue to be used directly for in vitro fertilization, as an alternative to surgical re-insertion of the tissue into the body.[14]
Empty follicle syndrome
IVM may also be an important consideration for female patients diagnosed with empty follicle syndrome (EFS). In EFS, no oocytes are retrieved from mature ovarian follicles despite the application of supraphysiological levels of gonadotrophins. A woman can be diagnosed with EFS after she has undergone multiple rounds of IVF with total (or near total) failure in each round.[21]
Rescue
Rescue IVM is a variant of classical in vitro maturation that involves attempting to mature immature oocytes that have been removed from a patient secondary to ovarian hyperstimulation in standard IVF practice. Therefore, allowing for more oocytes to mature to the developmental stage where they can be developmentally viable. However, rescue IVM has been considered a controversial field: If oocytes have not matured sufficiently in vivo – despite exposure to significant levels of gonadotrophins – it may be indicative of dysmaturity and of a limited potential developmentally.[19]
In animals
IVM has also been used in domestic animals including mice,[24] cats,[25][26] dogs,[27][28] swine,[29] sheep,[30] horse[31] and cattle[32][33] as well as wild species such as buffalo,[34] bison,[35] fish,[36] lions,[37] tigers[37] and leopards.[37] The ability to recover animals' oocytes initially destined for ovarian follicle atresia, can be utilized by researchers, conservationists and the agriculture industry for academic purposes or for improving breeding systems.
In research, IVM can be carried out on animals so as to understand the developmental capacities of oocytes under certain conditions, or to understand the specific reproductive biology during that developmental period. IVM in other species is also carried out as some animals are used as models to study human-related reproductive biology.[38] This research is often carried out with the aim of improving success rates of in vitro systems and/or aim to improve fertility in vivo.
Animal IVM models can be used to study how different exposures to harmful and toxic substances affect the maturing oocytes and their ability to become fertilized and develop into an embryo.[39]
It can also be used for subsequent biotechnology applications such as for the creation of transgenic animals using innovative gene-editing techniques such as CRISPR/Cas9, TALENs and ZFNs for biomedical research. An example includes genetically engineered pigs with CD163 and CD1D genes knocked out.[40] One of the ways these pigs were created was by injecting the CRISPR/Cas9 system into fertilised oocytes that were matured in vitro.
In agriculture, IVM is usually carried out prior to IVF or artificial insemination as a means of conserving desirable traits of particular animals within herds and counteracting lower production as a result of seasonal breeding. In livestock species such cattle, transvaginal oocyte recovery from the ovaries of live female animals can be repeatedly carried out prior to the in vitro production of embryos.[41]
In non-domesticated animals, IVM can also be used for the conservation of endangered species whilst maintaining genetic diversity.[42] However, due to limited resources and the species-specific nature of assisted reproductive technologies, the application of techniques such as IVM is still rare for non-domesticated animals.[42]
Success rate and future uses
In an experiment by Segers I et al. (2015), the overall maturation rate after IVM of oocytes recovered from ovariectomy specimens in laboratory was 36%. The maturation rate correlated with the age of patient and duration of IVM. With the 8 couples with embryo cryopreservation, there was a 65% fertilisation rate. At least one good quality day 3 embryo was cryopreserved in 7/8 couples. This experiment shows that IVM of oocytes obtained ex vivo during the processing of ovarian cortex prior to cryopreservation is a promising solution for patients at risk for fertility loss.[43]
The success of embryo production in vitro depends upon the use of an efficient oocyte retrieval technique and the best results have been obtained by laparoscopic aspiration.[44]
Limitations
The obstetric and perinatal outcomes of births from IVM cycles are similar to those with ICSI treatments.[45] However, IVM involves the use of invasive techniques, this may harm the mother. Furthermore, embryological outcome of IVM is not established.[46] A more comprehensive appraisal of health status of IVM children will demand larger prospective studies.[45] The potential of cryopreserved IVM oocytes from cancer patients remain unknown. The optimal number of IVM oocytes frozen in candidates for fertility preservation (FP) is unknown. FP oocytes of infertile PCOS women have decreased competence compared to oocytes recovered after ovarian stimulation. The FP strategy of cryopreservation of oocytes after IVM should only be considered should ovarian stimulation is unfeasible.[47]
In norma-ovulatory women, the success rate of IVM is lower than conventional ovarian stimulation regimens with poorer implantation and pregnancy rates. IVM is suboptimal and influenced by several factors. However, IVM is a milder approach to assisted reproduction treatment and an alternative procedure for specific conditions. Accurate patient selection can improve IVM clinical outcome.[45]
Improvements
IVM of oocytes cryopreserved may assist urgent fertility preservation in cancer patients. However, there is insufficient data regarding this outcome. Improving the culture conditions may increase the maturation rates and the potential of IVM oocytes.[48]
Besides that, in mouse oocytes, I-Carnitine (LC) supplementation during vitrification of germinal vesicle (GV) and their subsequent IVM improved nuclear maturation as well as meiotic spindle assembly and mitochondrial distribution in MII oocytes. However, no data to date has proven this benefit in fetal development and birth of healthy offspring after embryo transfer to surrogate females. However, this protocol could potentially improve the quality of vitrified human oocytes and embryos during IVM.[49] In a research by Wang X et al. (2014), gonadotropins affect oocyte maturation, fertilisation and developmental competence in vitro. The responsiveness of bovine oocytes to gonadotropins in vitro depends on the relative concentrations (FSH/LH) for optimal oocyte development developmental competence. Optimal FSH/LH concentrations could improve therapeutic clinical stimulation protocols and IVF success rates.[50]
References
- ^ a b Pincus G, Enzmann EV (October 1935). "The Comparative Behavior of Mammalian Eggs in Vivo and in Vitro: I. The Activation of Ovarian Eggs". The Journal of Experimental Medicine. 62 (5): 665–75. doi:10.1084/jem.62.5.665. PMC 2133299. PMID 19870440.
- ^ Edwards RG (October 1965). "Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes". Nature. 208 (5008): 349–51. Bibcode:1965Natur.208..349E. doi:10.1038/208349a0. PMID 4957259. S2CID 4285338.
- ^ a b Edwards RG (November 1965). "Maturation in vitro of human ovarian oöcytes". Lancet. 2 (7419): 926–9. doi:10.1016/s0140-6736(65)92903-x. PMID 4165802.
- ^ Cha KY, Koo JJ, Ko JJ, Choi DH, Han SY, Yoon TK (January 1991). "Pregnancy after in vitro fertilization of human follicular oocytes collected from nonstimulated cycles, their culture in vitro and their transfer in a donor oocyte program". Fertility and Sterility. 55 (1): 109–13. doi:10.1016/s0015-0282(16)54068-0. PMID 1986950.
- ^ Trounson A, Wood C, Kausche A (August 1994). "In vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patients". Fertility and Sterility. 62 (2): 353–62. doi:10.1016/S0015-0282(16)56891-5. PMID 8034085.
- ^ a b c Dunlop CE, Anderson RA (2014-08-01). "The regulation and assessment of follicular growth". Scandinavian Journal of Clinical and Laboratory Investigation. Supplementum. 244 (sup244): 13–7, discussion 17. doi:10.3109/00365513.2014.936674. PMID 25083887. S2CID 13731131.
- ^ a b c Chian RC, Lim JH, Tan SL (June 2004). "State of the art in in-vitro oocyte maturation". Current Opinion in Obstetrics & Gynecology. 16 (3): 211–9. doi:10.1097/00001703-200406000-00003. PMID 15129050. S2CID 46231802.
- ^ a b c Hardy K, Wright CS, Franks S, Winston RM (2000). "In vitro maturation of oocytes". British Medical Bulletin. 56 (3): 588–602. doi:10.1258/0007142001903391. PMID 11255547.
- ^ a b Chang EM, Song HS, Lee DR, Lee WS, Yoon TK (June 2014). "In vitro maturation of human oocytes: Its role in infertility treatment and new possibilities". Clinical and Experimental Reproductive Medicine. 41 (2): 41–6. doi:10.5653/cerm.2014.41.2.41. PMC 4102689. PMID 25045627.
- ^ a b c d "IVF Worldwide". In Vitro Maturation. Retrieved 2016-09-27.
- ^ Son WY, Tan SL (2010). "Laboratory and embryological aspects of hCG-primed in vitro maturation cycles for patients with polycystic ovaries". Human Reproduction Update. 16 (6): 675–89. doi:10.1093/humupd/dmq014. PMID 20504873.
- ^ a b Wani NA, Wani GM, Khan MZ, Salahudin S (2000). "Effect of oocyte harvesting techniques on in vitro maturation and in vitro fertilization in sheep". Small Ruminant Research. 36 (1): 63–67. doi:10.1016/s0921-4488(99)00097-8.
- ^ Niwa K (1993). "Effectiveness of in vitro maturation and in vitro fertilization techniques in pigs". Journal of Reproduction and Fertility. Supplement. 48: 49–59. PMID 8145214.
- ^ a b
- McLaughlin M, Albertini DF, Wallace WH, Anderson RA, Telfer EE (March 2018). "Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system". Molecular Human Reproduction. 24 (3): 135–142. doi:10.1093/molehr/gay002. hdl:20.500.11820/55cbb793-3401-4172-80a9-44412ecdf216. PMID 29390119.
- Further comments in BBC News article: Gallagher J (2018-02-09). "First human eggs grown in laboratory". BBC News.
- ^ Hyun CS, Cha JH, Son WY, Yoon SH, Kim KA, Lim JH (July 2007). "Optimal ICSI timing after the first polar body extrusion in in vitro matured human oocytes". Human Reproduction. 22 (7): 1991–5. doi:10.1093/humrep/dem124. PMID 17513319.
- ^ a b Hardy K, Wright CS, Franks S, Winston RM (2000-01-01). "In vitro maturation of oocytes". British Medical Bulletin. 56 (3): 588–602. doi:10.1258/0007142001903391. PMID 11255547.
- ^ Kannan S, Mehta A, Simha V, Reddy OK, Kaur BP, Onteru SK, Singh D (2014). "Photoinduction of granulosa cell and oocyte co-culture to influence in vitro maturation and fertilisation". Hypothesis. 12 (1): e7.
- ^ a b Khalili MA, Dehghan M, Nazari S, Agha-Rahimi A (March 2014). "Assessment of ovarian tissues autografted to various body sites followed by IVM in mouse". Iranian Journal of Reproductive Medicine. 12 (3): 199–204. PMC 4009574. PMID 24799880.
- ^ a b c d e f g h i j Vitek W, Robins JC (2013-10-01). "In vitro maturation". The Obstetrician & Gynaecologist. 15 (4): 215–219. doi:10.1111/tog.12050. ISSN 1744-4667.
- ^ a b c Ouandaogo ZG, Assou S, Haouzi D, Anahory T, Dechaud H, Hamamah S (2010). "Gene expression profile in human cumulus cells of immature and mature oocyte under in vivo maturation: clinical applications". Fertility and Sterility. 94 (4): S88. doi:10.1016/j.fertnstert.2010.07.338.
- ^ a b c d e f g Lee JE, Kim SD, Jee BC, Suh CS, Kim SH (December 2011). "Oocyte maturity in repeated ovarian stimulation". Clinical and Experimental Reproductive Medicine. 38 (4): 234–7. doi:10.5653/cerm.2011.38.4.234. PMC 3283078. PMID 22384448.
- ^ Dunaif A (December 1997). "Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis". Endocrine Reviews. 18 (6): 774–800. doi:10.1210/edrv.18.6.0318. PMID 9408743.
- ^ Shalom-Paz E, Holzer H, Son W, Levin I, Tan SL, Almog B (November 2012). "PCOS patients can benefit from in vitro maturation (IVM) of oocytes". European Journal of Obstetrics, Gynecology, and Reproductive Biology. 165 (1): 53–6. doi:10.1016/j.ejogrb.2012.07.001. PMID 22819571.
- ^ Martín-Coello J, González R, Crespo C, Gomendio M, Roldan ER (October 2008). "Superovulation and in vitro oocyte maturation in three species of mice (Mus musculus, Mus spretus and Mus spicilegus)". Theriogenology. 70 (6): 1004–13. doi:10.1016/j.theriogenology.2008.06.002. PMID 18640710.
- ^ Johnston LA, O'Brien SJ, Wildt DE (November 1989). "In vitro maturation and fertilization of domestic cat follicular oocytes". Gamete Research. 24 (3): 343–56. doi:10.1002/mrd.1120240310. PMID 2599509.
- ^ Goodrowe KL, Hay M, King WA (September 1991). "Nuclear maturation of domestic cat ovarian oocytes in vitro". Biology of Reproduction. 45 (3): 466–70. doi:10.1095/biolreprod45.3.466. PMID 1782295.
- ^ Mahi CA, Yanagimachi R (May 1976). "Maturation and sperm penetration of canine ovarian oocytes in vitro". The Journal of Experimental Zoology. 196 (2): 189–96. Bibcode:1976JEZ...196..189M. doi:10.1002/jez.1401960206. PMID 1271036.
- ^ Nickson DA, Boyd JS, Eckersall PD, Ferguson JM, Harvey MJ, Renton JP (1993-01-01). "Molecular biological methods for monitoring oocyte maturation and in vitro fertilization in bitches". Journal of Reproduction and Fertility. Supplement. 47: 231–40. PMID 8229931.
- ^ Motlik J, Crozet N, Fulka J (November 1984). "Meiotic competence in vitro of pig oocytes isolated from early antral follicles". Journal of Reproduction and Fertility. 72 (2): 323–8. doi:10.1530/jrf.0.0720323. PMID 6392543.
- ^ Szöllösi D, Desmedt V, Crozet N, Brender C (1988-01-01). "In vitro maturation of sheep ovarian oocytes". Reproduction, Nutrition, Development. 28 (4B): 1047–80. doi:10.1051/rnd:19880705. PMID 3244901.
- ^ Squires EL (April 1996). "Maturation and fertilization of equine oocytes". The Veterinary Clinics of North America. Equine Practice. 12 (1): 31–45. doi:10.1016/S0749-0739(17)30293-6. PMID 8726448.
- ^ Hensleigh HC, Hunter AG (June 1985). "In vitro maturation of bovine cumulus enclosed primary oocytes and their subsequent in vitro fertilization and cleavage". Journal of Dairy Science. 68 (6): 1456–62. doi:10.3168/jds.S0022-0302(85)80983-8. PMID 3926843.
- ^ Barile VL, Dell'Aquila ME, Cinone M, Minoia P (September 1990). "In vitro maturation and fertilization of follicular oocytes in cattle". Bollettino della Societa Italiana di Biologia Sperimentale. 66 (9): 899–906. PMID 2073391.
- ^ Totey SM, Singh G, Taneja M, Pawshe CH, Talwar GP (July 1992). "In vitro maturation, fertilization and development of follicular oocytes from buffalo (Bubalus bubalis)". Journal of Reproduction and Fertility. 95 (2): 597–607. doi:10.1530/jrf.0.0950597. PMID 1518014.
- ^ Cervantes MP, Palomino JM, Anzar M, Mapletoft RJ, Adams GP (October 2016). "In vivo and in vitro maturation of oocytes collected from superstimulated wood bison (Bison bison athabascae) during the anovulatory and ovulatory seasons". Animal Reproduction Science. 173: 87–96. doi:10.1016/j.anireprosci.2016.09.001. PMID 27601321.
- ^ Young G, Kagawa H, Nagahama Y (December 1982). "Oocyte maturation in the amago salmon (Oncorhynchus rhodurus): in vitro effects of salmon gonadotropin, steroids, and cyanoketone (an inhibitor of 3 beta-hydroxy-delta 5-steroid dehydrogenase)". The Journal of Experimental Zoology. 224 (2): 265–75. Bibcode:1982JEZ...224..265Y. doi:10.1002/jez.1402240217. PMID 6961189.
- ^ a b c Rao BS, Mahesh YU, Suman K, Charan KV, Nath R, Rao KR (January 2015). "Meiotic maturation of oocytes recovered from the ovaries of Indian big cats at postmortem". In Vitro Cellular & Developmental Biology. Animal. 51 (1): 19–25. doi:10.1007/s11626-014-9802-x. PMID 25124872. S2CID 16289775.
- ^ Nikmard F, Hosseini E, Bakhtiyari M, Ashrafi M, Amidi F, Aflatoonian R (April 2017). "Effects of melatonin on oocyte maturation in PCOS mouse model". Animal Science Journal. 88 (4): 586–592. doi:10.1111/asj.12675. PMID 27530294.
- ^ Hallberg, Ida; Persson, Sara; Olovsson, Matts; Moberg, Mikaela; Ranefall, Petter; Laskowski, Denise; Damdimopoulou, Pauliina; Sirard, Marc-Andre; Rüegg, Joëlle; Sjunnesson, Ylva C.B. (April 2022). "Bovine oocyte exposure to perfluorohexane sulfonate (PFHxS) induces phenotypic, transcriptomic, and DNA methylation changes in resulting embryos in vitro". Reproductive Toxicology. 109: 19–30. doi:10.1016/j.reprotox.2022.02.004.
- ^ Whitworth KM, Lee K, Benne JA, Beaton BP, Spate LD, Murphy SL, et al. (September 2014). "Use of the CRISPR/Cas9 system to produce genetically engineered pigs from in vitro-derived oocytes and embryos". Biology of Reproduction. 91 (3): 78. doi:10.1095/biolreprod.114.121723. PMC 4435063. PMID 25100712.
- ^ Lonergan P, Fair T (2016-01-01). "Maturation of Oocytes in Vitro". Annual Review of Animal Biosciences. 4: 255–68. doi:10.1146/annurev-animal-022114-110822. PMID 26566159.
- ^ a b Andrabi SM, Maxwell WM (June 2007). "A review on reproductive biotechnologies for conservation of endangered mammalian species". Animal Reproduction Science. 99 (3–4): 223–43. doi:10.1016/j.anireprosci.2006.07.002. PMID 16919407.
- ^ Segers I, Mateizel I, Van Moer E, Smitz J, Tournaye H, Verheyen G, De Vos M (August 2015). "In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: a promising "ex vivo" method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe". Journal of Assisted Reproduction and Genetics. 32 (8): 1221–31. doi:10.1007/s10815-015-0528-9. PMC 4554385. PMID 26253691.
- ^ Padilha LC, Teixeira PP, Pires-Buttler EA, Apparício M, Motheo TF, Savi PA, et al. (April 2014). "In vitro maturation of oocytes from Santa Ines ewes subjected to consecutive sessions of follicular aspiration by laparoscopy". Reproduction in Domestic Animals = Zuchthygiene. 49 (2): 243–8. doi:10.1111/rda.12261. PMID 24313350.
- ^ a b c Fadini R, Mignini Renzini M, Guarnieri T, Dal Canto M, De Ponti E, Sutcliffe A, et al. (December 2012). "Comparison of the obstetric and perinatal outcomes of children conceived from in vitro or in vivo matured oocytes in in vitro maturation treatments with births from conventional ICSI cycles". Human Reproduction. 27 (12): 3601–8. doi:10.1093/humrep/des359. PMID 23042796.
- ^ Sánchez F, Romero S, De Vos M, Verheyen G, Smitz J (June 2015). "Human cumulus-enclosed germinal vesicle oocytes from early antral follicles reveal heterogeneous cellular and molecular features associated with in vitro maturation capacity". Human Reproduction. 30 (6): 1396–409. doi:10.1093/humrep/dev083. PMID 25904637.
- ^ Sonigo C, Simon C, Boubaya M, Benoit A, Sifer C, Sermondade N, Grynberg M (July 2016). "What threshold values of antral follicle count and serum AMH levels should be considered for oocyte cryopreservation after in vitro maturation?". Human Reproduction. 31 (7): 1493–500. doi:10.1093/humrep/dew102. PMID 27165625.
- ^ Grynberg M, Poulain M, le Parco S, Sifer C, Fanchin R, Frydman N (March 2016). "Similar in vitro maturation rates of oocytes retrieved during the follicular or luteal phase offer flexible options for urgent fertility preservation in breast cancer patients". Human Reproduction. 31 (3): 623–9. doi:10.1093/humrep/dev325. PMID 26759139.
- ^ Moawad AR, Xu B, Tan SL, Taketo T (October 2014). "l-carnitine supplementation during vitrification of mouse germinal vesicle stage-oocytes and their subsequent in vitro maturation improves meiotic spindle configuration and mitochondrial distribution in metaphase II oocytes". Human Reproduction. 29 (10): 2256–68. doi:10.1093/humrep/deu201. PMID 25113843.
- ^ Wang X, Tsai T, Qiao J, Zhang Z, Feng HL (June 2014). "Impact of gonadotropins on oocyte maturation, fertilisation and developmental competence in vitro". Reproduction, Fertility, and Development. 26 (5): 752–7. doi:10.1071/RD13024. PMID 23726536.
